Question
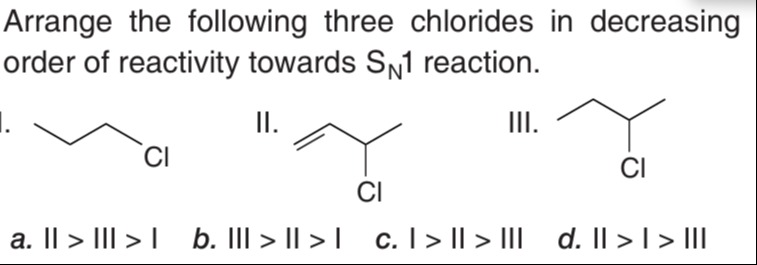
Question: Arrange the following three chlorides in decreasing order of reactivity towards $S_N1$ reaction. ...
Arrange the following three chlorides in decreasing order of reactivity towards SN1 reaction.
II > III > I
III > II > I
I > II > III
II > I > III
a. II > III > I
Solution
The SN1 reaction proceeds through the formation of a carbocation intermediate. The rate of the SN1 reaction is determined by the stability of the carbocation formed. The more stable the carbocation, the faster the SN1 reaction. We need to determine the stability of the carbocations formed from the ionization of the given chlorides.
I. 1-chloropropane: CH3−CH2−CH2−Cl. Upon ionization, it forms a primary carbocation: CH3−CH2−CH2+.
II. 3-chloro-1-propene (Allyl chloride): CH2=CH−CH2−Cl. Upon ionization, it forms an allyl carbocation: CH2=CH−CH2+. This carbocation is stabilized by resonance: CH2=CH−CH2+↔CH2+−CH=CH2.
III. 2-chloropropane (Isopropyl chloride): CH3−CH(Cl)−CH3. Upon ionization, it forms a secondary carbocation: CH3−CH+−CH3. This carbocation is stabilized by hyperconjugation from the two methyl groups (6 alpha hydrogens).
Comparing the stability of the carbocations:
Primary carbocations are generally the least stable among simple alkyl carbocations.
Secondary carbocations are more stable than primary carbocations due to hyperconjugation and inductive effects.
Allyl carbocations are stabilized by resonance, which is a powerful stabilizing effect. Resonance stabilization of allyl carbocation is generally greater than hyperconjugation stabilization of a simple secondary carbocation.
Therefore, the order of stability of the carbocations is: Allyl carbocation (from II) > Secondary carbocation (from III) > Primary carbocation (from I).
Stability order: II > III > I.
Since the reactivity towards SN1 reaction is directly proportional to the stability of the carbocation formed, the decreasing order of reactivity is the same as the decreasing order of carbocation stability.
Decreasing order of reactivity towards SN1: II > III > I.
