Question
Question: Arrange the following in the increasing order of their basic strength. \(\text{C}{{\text{H}}_{3}}...
Arrange the following in the increasing order of their basic strength.
CH3NH2 , (CH3)2NH , C6H5NH2 , C6H5CH2NH2
Solution
Basic strength means the ability to donate electrons to electrophiles (electron deficient species). Check the factors on which the basicity depends and their effect. Here, CH3NH2 and (CH3)2NH is aliphatic amines and C6H5NH2 and C6H5CH2NH2 are aromatic amines.
Complete step by step answer:
Let us discuss the factors on which basic strength depends to get the general order of the bases:
| S. No. | Factors of basicity | Definitions | Effect on basic strength | Reason of that effect |
|---|---|---|---|---|
| 1. | Inductive effect | It is displacement of sigma electrons due to electronegativity or electropositivity of elements. | Positive inductive effect increases the basicity and Negative inductive effect decreases the basicity | More the positive inductive effect (donation of electrons) or +I effect which increases the electron density on the molecule. Due to which electron donating ability increases of a molecule, making it basic. Number of +I groups on a molecule increases, its basicity increases. Whereas, −I effect decreases the electron density on the molecule making it electron deficient reducing the basicity. Number of −I groups attached increases, its basic strength decreases. Hence, order of basicity is tertiary> secondary> primary. As methyl groups attached produce +Ieffect. |
| 2. | Resonance | Resonance is actually the delocalization or movement of π electrons. | Decreases the basicity | The delocalization of π electrons makes it difficult for nitrogen to share its lone pair. Thus, the basic strength of compound decreases. Hence, aromatic amines are less basic than aliphatic amines. |
A. Methyl amine: It has one methyl group attached to it and is aliphatic in nature. It is basic in nature.
B. Dimethyl amine: It has two methyl groups attached to it and is aliphatic in nature. It is highly basic.
C. Aniline: It has no methyl group attached. It is an aromatic amine in which −NH2 group is attached to a benzene ring. The lone pair of −NH2 group undergoes resonance with the benzene ring making it less basic.
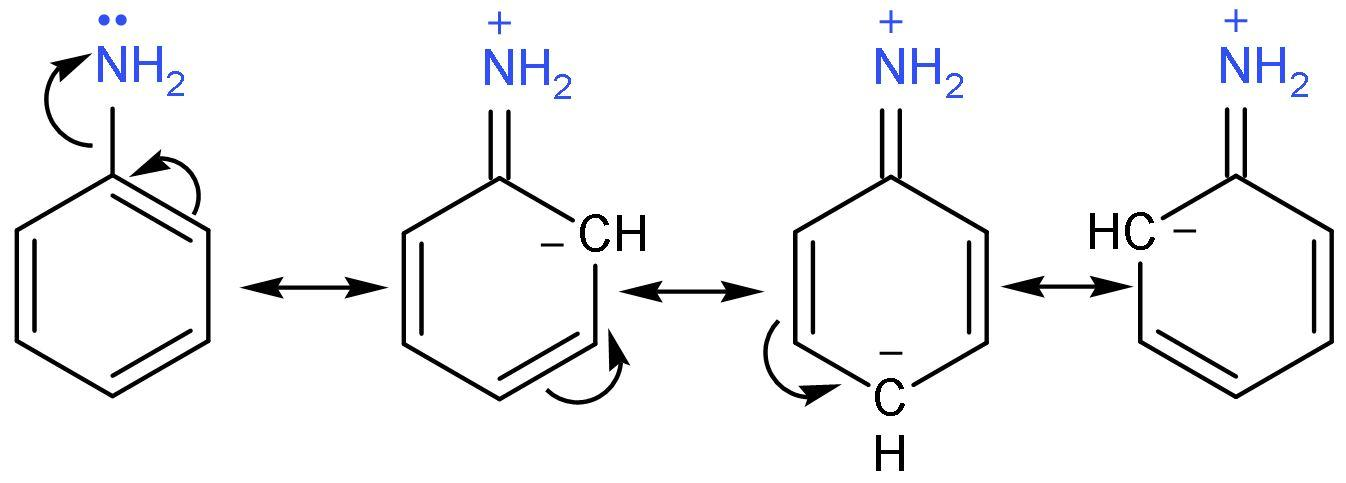
D. Benzyl amine: It is primary amine and lone pair of nitrogen does not undergo resonance with benzene ring due to (−CH2−) group in between the two. The −I effect of phenyl group decreases the basicity.
The increasing order of their basic strength is (CH3)2NH>CH3NH2>C6H5CH2NH2>C6H5NH2.
Note: In aqueous solution, the order of basic strength is (CH3)2NH>CH3NH2>(CH3)3N. The order mainly changes due to solvation effect (interaction between solute and solvent, here cations), inductive effect and steric hindrance (crowd of groups).
