Question
Question: Arrange the following in decreasing order of nucleophilicity: 1.\[{\text{C}}{{\text{H}}_{\text{3}}...
Arrange the following in decreasing order of nucleophilicity:
1.CH3COO -
2.CH3O -
3.CN -
4.Ref. image
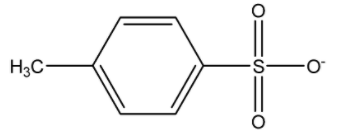
A. 3, 2, 1, 4
B. 1, 2, 3, 4
C. 4, 3, 2, 1
D. 2, 3, 1, 4
Solution
The nucleophilicity of a chemical species can be defined as the ability of that species (which is nucleophilic in nature) to displace a leaving group in a substitution reaction. Weaker the conjugate acids of the nucleophiles, stronger the nucleophile.
Complete step-by-step answer:
The nucleophilicity of the ligands depend on a number of factors. The term “nucleophilic” describes the affinity of a nucleophile for a positively charged nucleus. The nucleophilicity should not be confused with the definition of basicity. The nucleophilicity depends on the strength of the conjugate acid of the nucleophile. If the conjugate acid of the nucleophile is a weak acid, then the corresponding nucleophile will be stronger in nature.
Going by that fact, the conjugate acids of the above nucleophiles are acetic acid, methanol, hydrogen cyanide and toluene para sulphonic acid. Among the following acids, the weakest acid is methanol. Hence its conjugate base, methoxy group will be the strongest nucleophile, while the weakest one will be the p-toluene sulphonate.
Hence, the correct order of the nucleophilicity among the given is: 2, 3, 1, 4, i.e. option D.
Note: The strength of the nucleophiles also depends on the steric hindrance and the structure of the molecules. The more a compound is bulky, the lesser will be the nucleophilicity. Hence as the para toluene sulphonate is a bulky group so it is a bad nucleophile but it is a good leaving group for the same reason.
