Question
Question: Arrange the following in a proper sequence for writing the structural formula from the given IUPAC n...
Arrange the following in a proper sequence for writing the structural formula from the given IUPAC name 4−methyl−4−nonene.
(a) Locate the primary suffix and insert at the given position.
(b) Identify the substituent and insert at the given position.
(c) Add a suitable number of hydrogen atoms to satisfy the valencies of each carbon atom.
(d) Identify the root word and write down the parent carbon chain.
A) dacb
B) bdac
C) dabc
D) dcba
Solution
Before writing the structure we should be familiar with the rules and regulations established by IUPAC for writing the name of an organic compound.
In the given IUPAC name the root word is ‘non’ and the primary suffix is ‘ene’ implying that the given compound will be an alkene having nine carbon in the straight chain or parent carbon chain
Also one should be familiar with methyl which is a substituent obtained from methane molecules by elimination of one Hydrogen atom.
Complete step by step answer:
To write the structure of the given compound the step one should be writing a straight chain of carbon referring to the root word which is ‘non’ indicating presence of nine carbon atom in the straight chain or the parent carbon chain as shown below
C−C−C−C−C−C−C−C−C
The step two should be to find out the primary suffix which is ‘ene’ here and its position is given as four for the given compound indicating a double bond at position four hence the structure further become as follow
C−C−C−C=C−C−C−C−C
Now the third step should be to identify the substituent group which is methyl (−CH3) here which is formed by elimination of one hydrogen atom from methane (CH4)
The position of methyl is given as four applying this step makes the structure as follow
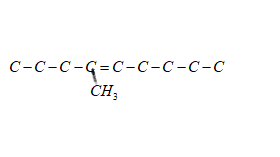
Finally the fourth and last step is to satisfy the valencies of each atom adding suitable number of hydrogen atoms (using the fact carbon has tetravalency)
We get the final structure of 4−methyl−4−nonene.
as follow
CH3−CH2−CH2−(CH3)C=CH−CH2−CH2−CH2−CH3
Hence the steps should be in the order of dabc
Hence option ‘C’ is the correct solution for the given question.
Note:
The given structure of the compound can also be name as follow
4−methyl−non−4−ene Which is also valid on the basis of rules and regulations of IUPAC naming
Also remember that root word for nine carbon atom parent chain is “nona” but due to presence of a vowel ‘e’ of ‘ene’ adjacent to ‘a’ of ‘nona’ we remove the letter ‘a’ while writing the IUPAC name of the given compound
