Question
Question: Arrange the following elements in order of decreasing metallic character: \[Sc,\text{ }Fe,\text{ }Rb...
Arrange the following elements in order of decreasing metallic character: Sc, Fe, Rb, Br, O, Ca, F, Te
A. Rb > Ca > Sc > Fe > Te > Br > O > F
B. Ca >Rb > Sc > Fe > Br > Te > O > F
C. Rb > Sc > Ca > Fe > Te > Br > F > O
D. None of these
Solution
Metallic character is a periodic trend which could be observed in periodic tables.
The more loosely bound the electron is, the more metallic character it will have.
Complete step by step answer:
-Metallic character denotes the extent of the reactivity of a metal. In chemical reactions, metals tend to lose electrons, as we can see by their low ionization energies. Within a compound, metal atoms relatively attract electrons in a more lenient way, as indicated by their low electronegativities.
-Non-metals tend to gain electrons in chemical reactions and have a high affinity for electrons inside a compound. The most reactive non-metals exist in the upper right portion of the periodic table. As we know, the noble gases are a special kind of group because of their lack of reactivity, the element fluorine is the most reactive non-metal. 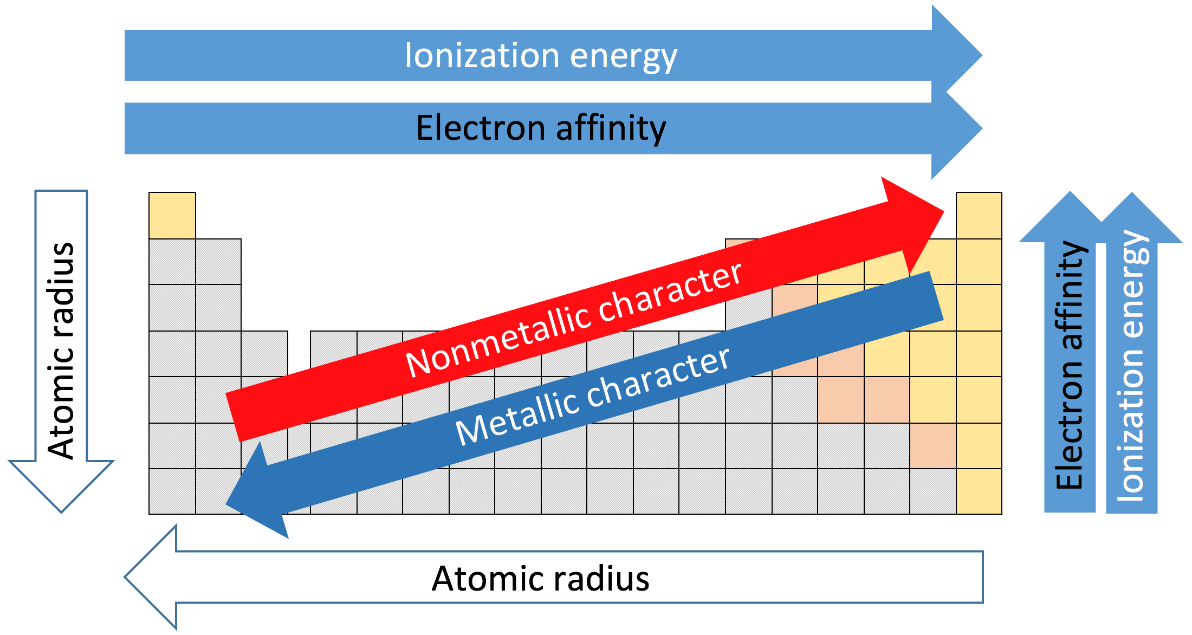
-The nuclear charge experienced by electrons, after it gets shielded by the other electrons, is called effective nuclear charge.
-From the figure above, we can see that, as we move from left to right across a period the metallic character decreases. This is because, when moving from left to right, one electron gets added in the same shell, and the effective nuclear charge experienced by each electron also increases as one neutron is also added to the nucleus. So, the ionisation energy also increases across a period, as it becomes difficult to separate the outer electrons from an isolated gaseous atom.
-Similarly, from the diagram we can see that, as we move from top to bottom in a group, the metallic character increases, as one more shell gets added each time we go down a group and the outermost electrons experience lesser effective nuclear charge, so it becomes easier to eject an electron from the outermost shell.
So the correct answer is Rb > Ca > Sc > Fe > Te > Br > O > F.
Note:
When dealing with the metallic character of a compound, look for the ionisation enthalpy, as it can also give an idea about the metallic character of the element. if the element has higher ionisation enthalpy then it will have lower metallic character and vice versa.
