Question
Question: Arrange the following compounds in the increasing order of their melting points. ...
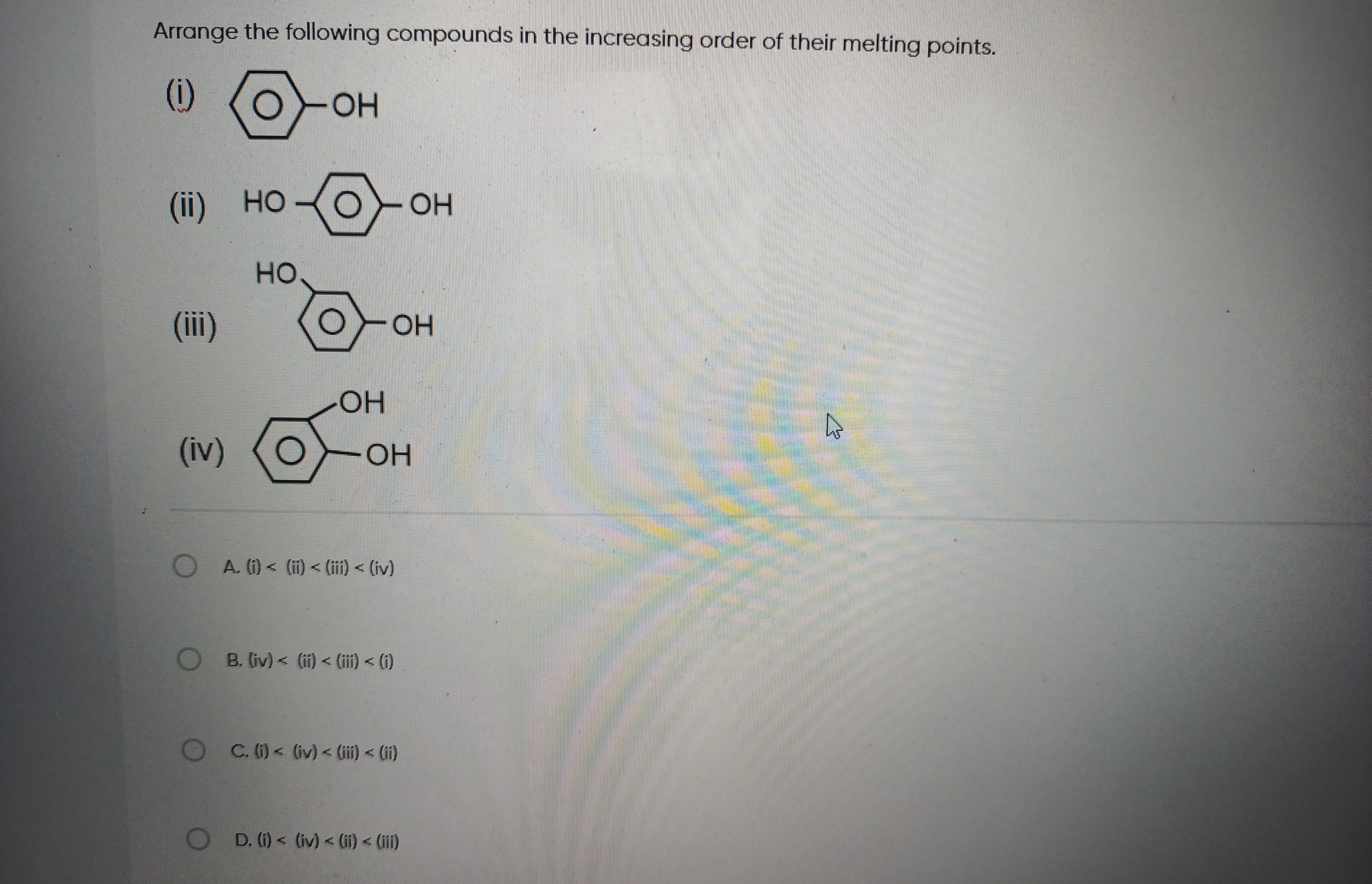
Arrange the following compounds in the increasing order of their melting points.
A
(i) < (ii) < (iii) < (iv)
B
(iv) < (ii) < (iii) < (i)
C
(i) < (iv) < (iii) < (ii)
D
(i) < (iv) < (ii) < (iii)
Answer
The provided options are incorrect. The correct order is (i) < (iii) < (ii) < (iv).
Explanation
Solution
The melting point of organic compounds is primarily determined by the strength of intermolecular forces. For phenols, hydrogen bonding is the dominant intermolecular force.
- Phenol (i): Has one hydroxyl group, resulting in weaker intermolecular forces compared to dihydroxybenzenes.
- Dihydroxybenzenes (ii, iii, iv): Have two hydroxyl groups, leading to stronger intermolecular hydrogen bonding.
- Catechol (iii): Ortho-dihydroxybenzene. Exhibits significant intramolecular hydrogen bonding, which reduces the extent of intermolecular hydrogen bonding, thus lowering its melting point compared to its isomers.
- Resorcinol (ii): Meta-dihydroxybenzene. Has stronger intermolecular hydrogen bonding than catechol.
- Hydroquinone (iv): Para-dihydroxybenzene. Due to its high symmetry, it packs more efficiently in the crystal lattice, leading to the strongest intermolecular forces and the highest melting point among the dihydroxybenzenes.
Therefore, the correct order of increasing melting points is: Phenol (i) < Catechol (iii) < Resorcinol (ii) < Hydroquinone (iv).
None of the given options match this order.
