Question
Question: Arrange the following compounds in increasing order of boiling points . Propan-1-ol , butan-I-ol ...
Arrange the following compounds in increasing order of boiling points .
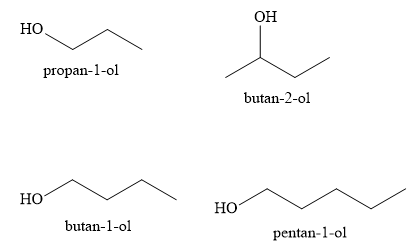
Propan-1-ol , butan-I-ol , butan-2-ol, pentan-1-ol.
A) Propan-l-ol, butan-2-ol,butan-1-ol ,pentan-1-ol
B) Propan-l-ol, butan-1-ol,butan-2-ol ,pentan-1-ol
C) Pentan-1-ol , butan-2-ol , butan-1-ol , propan -1-ol
D) Pentan-1-ol butan-1-ol butan-2-ol propan -1-ol
Solution
To answer this question let us consider the relationship between the molecular weight and the boiling point. Also consider the relationship between the degree of branching and the boiling point.
Complete answer:
The compounds Propan-l-ol, butan-2-ol, butan-1-ol ,pentan-1-ol belong to the alcohol family. Let us write the structure of these alcohols as shown below:
With increase in the molecular weight, the boiling points of alcohols increase. Hence, butan-1-ol has higher boiling point than propan-1-ol. Similarly, pentan-1-ol has higher boiling point than butan-1-ol.
Hence, the increasing order of the boiling points of alcohols is propan-1-ol < butan-1-ol < pentan-1-ol.
With increase in the branching of alkyl groups, the boiling point of alcohols decreases. Thus, butan-2-ol has lower boiling point than butan-1-ol. The increasing order of the boiling points of given alcohols is
Propan-l-ol < butan-2-ol < butan-1-ol < pentan-1-ol
Hence, the correct answer is the option (A)
Note: The boiling point of alcohols is much higher than the boiling point of alkanes. This is because alcohols form intermolecular hydrogen bonds which leads to molecular association. More energy is needed to break these intermolecular hydrogen bonds. A hydrogen bond is formed when a hydrogen atom is attached to electronegative nitrogen, oxygen or fluorine atom.