Question
Question: Arrange the following compounds in increasing order of C-OH bond length: methanol, phenol, p-ethox...
Arrange the following compounds in increasing order of C-OH bond length:
methanol, phenol, p-ethoxyphenol
(A)- phenol<methanol<p−ethoxyphenol
(B)- phenol<p−ethoxyphenol<methanol
(C)- methanol<phenol<p−ethoxyphenol
(D)- methanol<p−ethoxyphenol<phenol
Solution
The bond length of the given compounds can be determined by the partial double bond character strength due to the resonance effect by the substituents attached to the benzene ring.
Complete step by step answer:
In the given compounds, in order to determine the length of the C-OH bond, we will look into the bond strength due to the presence of partial double bond character between the C-O bond, in the compounds.
In the methanol compound, the resonance stabilisation does not occur. Only the positive inductive effect by the methyl group is seen, which does not contribute much in the strengthening of the bond. Thus, there is only a single bond present between the carbon and oxygen.
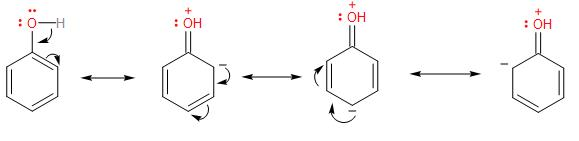
In the phenol compound, having the hydroxyl group (-OH) attached to the benzene ring, has a tendency to share its lone pair of electrons on the oxygen atom to the benzene ring, which get delocalised into the benzene ring through the resonance. In the resonance structures, a partial double-bond between C-O is formed, which strengthens the bond.
Whereas, in the p-ethoxyphenol compound, having an ethoxy- group in the para- position with respect to the -OH group, it shows both negative inductive effect (being an electron-withdrawing group) and the positive mesomeric effect.
In the mesomeric effect, it gives the lone pair of electrons to the benzene ring. In the stabilization of the charge through the resonance structures, a negative charge is formed on the carbon atom adjacent to the -OH group, which slightly decreases the stabilization and so the double- bond character. Thus, decreases the bond strength compared to the phenol molecule.
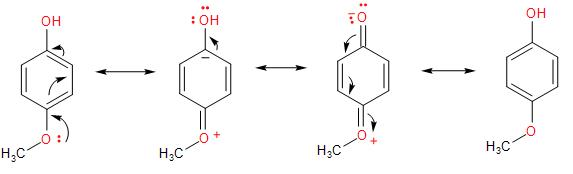
Therefore, the order of the C - O bond length in the given compounds is option (B)- phenol<p−ethoxyphenol<methanol.
So, the correct answer is “Option C”.
Note: With the presence of the double bond, the strength increases due to the resonance stabilizing structures. The bond length further helps in the determining the strength of the O-H bond and thus the acidity of the compounds.
