Question
Question: Arrange the following compounds according to the decreasing order of heat of combustion. a. i.Pe...
Arrange the following compounds according to the decreasing order of heat of combustion.
a.
i.Pentane
ii.Hexane
iii.2-methyl butane
iv.2,2-dimethylpropane
b. i. 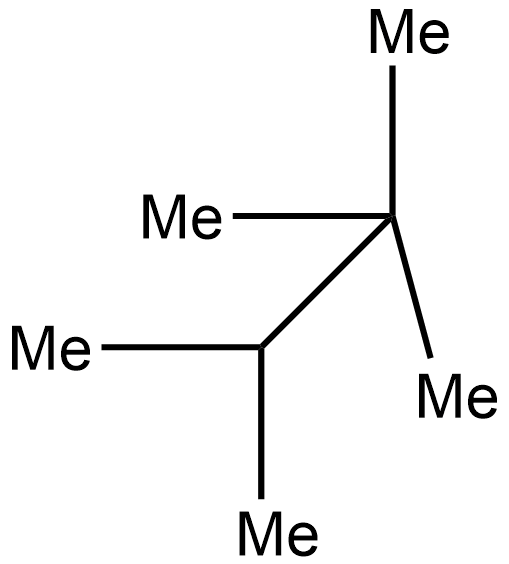
ii. 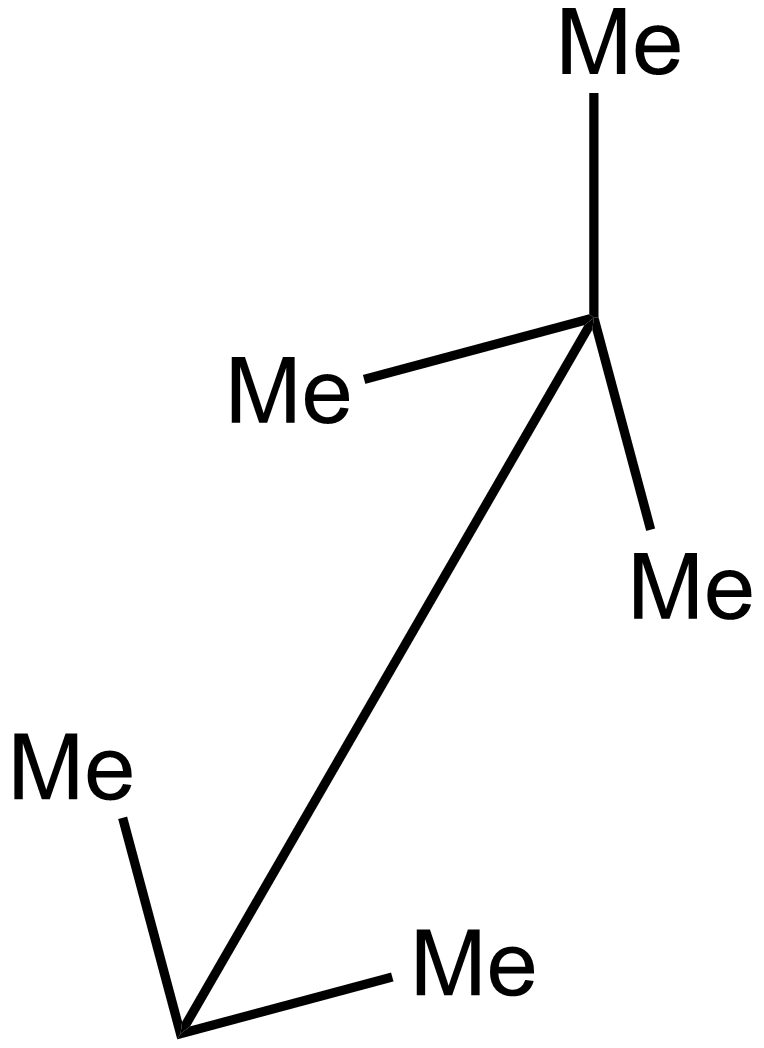
iii.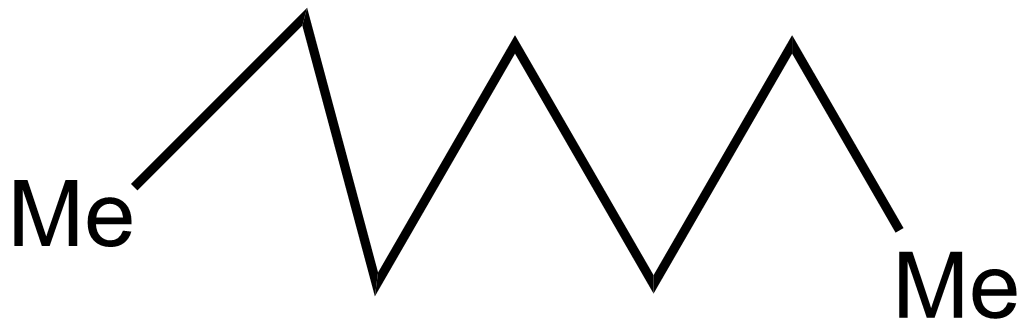
iv. 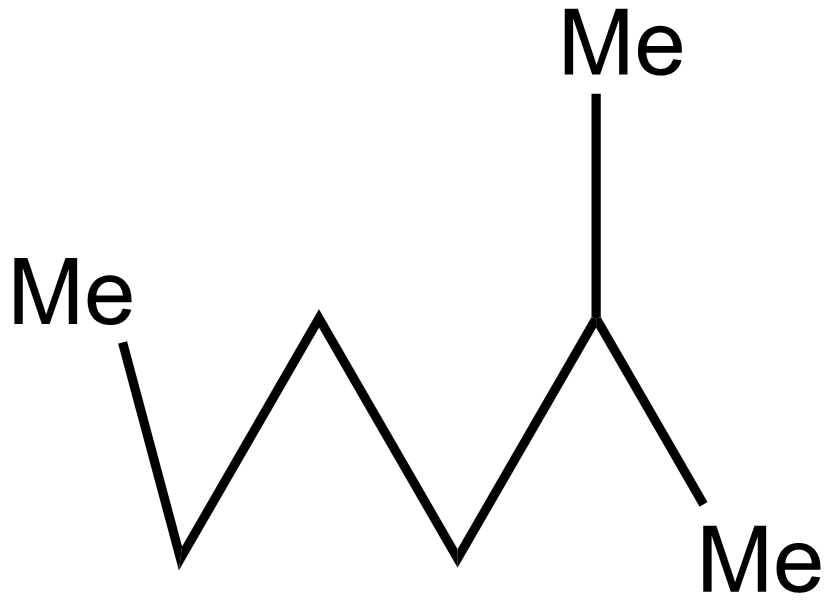
A.a: ii > iii > i > iv
b: ii > iv > i > iii
B.a: ii > iv > iii > i
b:iii > i > iv > ii
C.a: ii > i > iii > iv
b: iii > iv > i > ii
D.a: iv > iii > i > ii
b: ii > i > iv > iii
Solution
Heat of combustion is defined as the amount of heat evolved when one mole a substance is burnt in the presence of oxygen at constant volume. The heat of combustion is a negative value.
Complete step by step answer:
As we know the heat of combustion depends upon the number of carbon atoms present in the hydrocarbon chain. More will be the number of carbon atoms, more will be molecular mass and heat of combustion is also more. For the same number of carbon atoms, the heat of combustion for straight-chain hydrocarbons is more than the heat of combustion of a branched-chain hydrocarbon. This is because the surface area of straight-chain hydrogen carbon is more as compared to branched hydrocarbon.
If we talk about the first case, Hexane is a six-carbon straight-chain hydrocarbon whereas the pentane is a five-carbon straight-chain hydrocarbon. Since the number of carbon in hexane is more than that in pentane, as a result, the heat of combustion is greater than that of pentane, and if we talk about 2-methyl butane and 2,2-dimethyl propane both are branched-chain hydrocarbon. In the case of 2,2-dimethylpropane, branching is more as compared to 2-methyl butane. Hence the decreasing order of heat of combustion in the first case is:
Hexane>pentane>2-methyl butane>2,2-dimethylpropane.
In the 2nd case structure (iii) is a straight chain and the structure (i) is eclipsed and the structure(ii) is gauche. As we know gauche structure is more stable than eclipsed hence the decreasing order of heat of combustion is given as:
iii>iv>i>ii
Hence, option C is correct.
Note:
Heat of combustion is related to the stability of a compound also. More will be the stability, lesser will be the release of energy or we can say lesser will be the heat of combustion .i.e.
heat of combustion∝stability1
