Question
Question: Arrange the following acids in decreasing order of acidity . \(\left( X \right)\) 
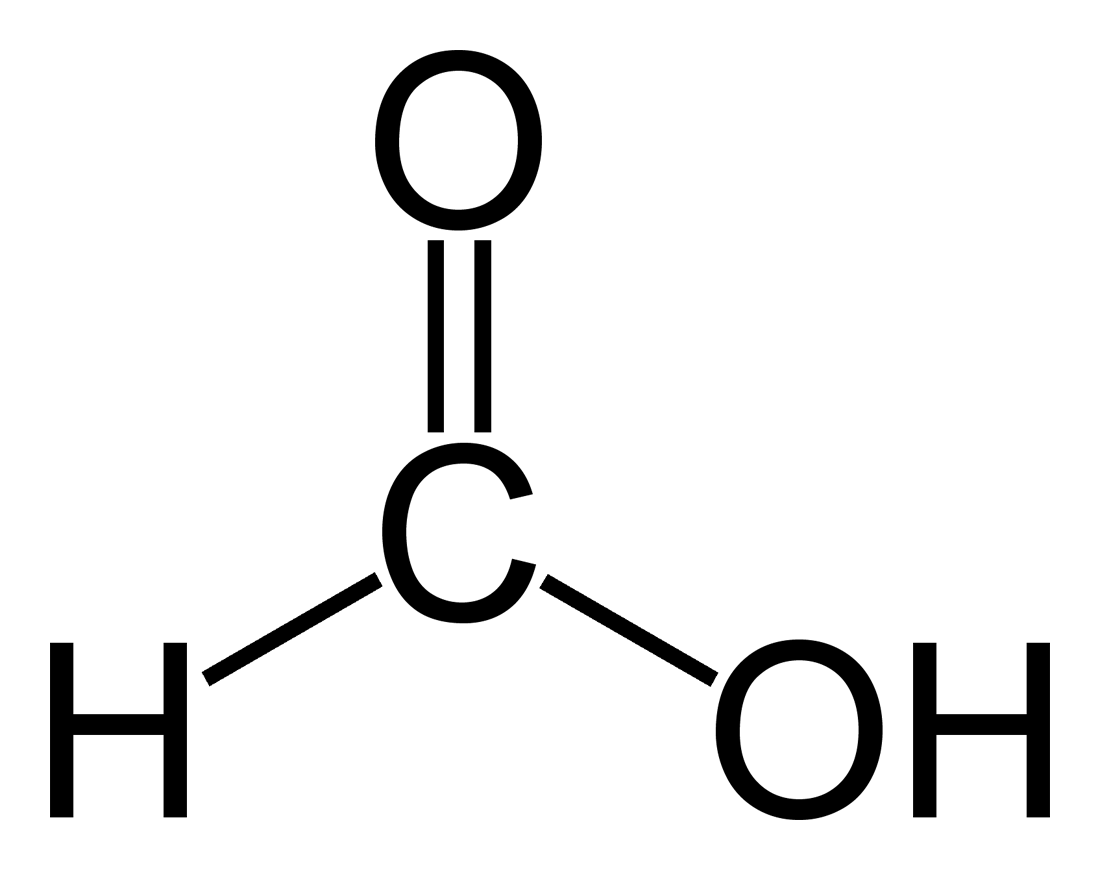
(Y)
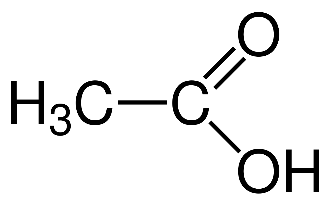
(Z)
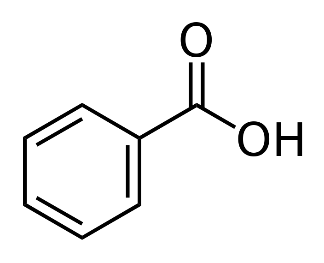
(a)X >Z >Y
(b)X >Y >Z
(c)Z >X >Y
(d)Z >Y >X
Solution
In the question we are given the different carboxylic acids ,the molecule which can release H+ ions easily are considered more acidic in nature . For this we need to check if the substituent groups are electron withdrawing groups or electron donating groups . If an electron withdrawing group is present then it will be more acidic in nature .
Complete step by step answer:
Carboxylic acids are acidic in nature because they release hydrogen when it gets dissociated . The hydrogen which is released belongs to the −COOH.
In benzoic acid , we have an electron withdrawing group which is stabilizing the conjugate base .
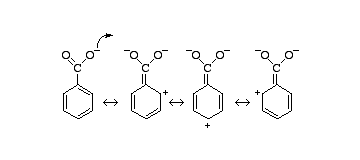
- The resonating structures of the conjugate base of benzoic acid makes the molecule more stable due to this it can easily donate the hydrogen and become more acidic .
- While in the acetic acid the electron donating group destabilizes it most , because the electron donating group increases the electron density and the oxygen atom already has an extra electron. So it will be less acidic compared to benzoic acid .
so , we can arrange the acids in decreasing order of acidity
Z >X >Y
The correct answer is option “C” .
Additional Information : Carboxylic acids are more acidic than other organic compounds but are less acidic than mineral acids . The conjugate base of carboxylic acid forms two resonating structures in which the negative charge is delocalised between two oxygen atoms . When the carboxylic acids react with metal they tend to form conjugated bases which are then stabilized by resonance .
Note: Remember the following electron withdrawing groups
Nitro groups, Aldehydes , Ketones , Esters , Cyano groups
- The strongest electron group is the one which contains pi bonds . electron withdrawing groups decrease the density in a molecule .
- Following are the electron donating groups
Alcohol groups, ethers , amine groups , the oxygen anion , alkyl groups .
- Electron donating groups make the molecule nucleophile because the centre becomes rich with electrons .
