Question
Question: Arrange in the order of decreasing \(p{K_b}\). (A)  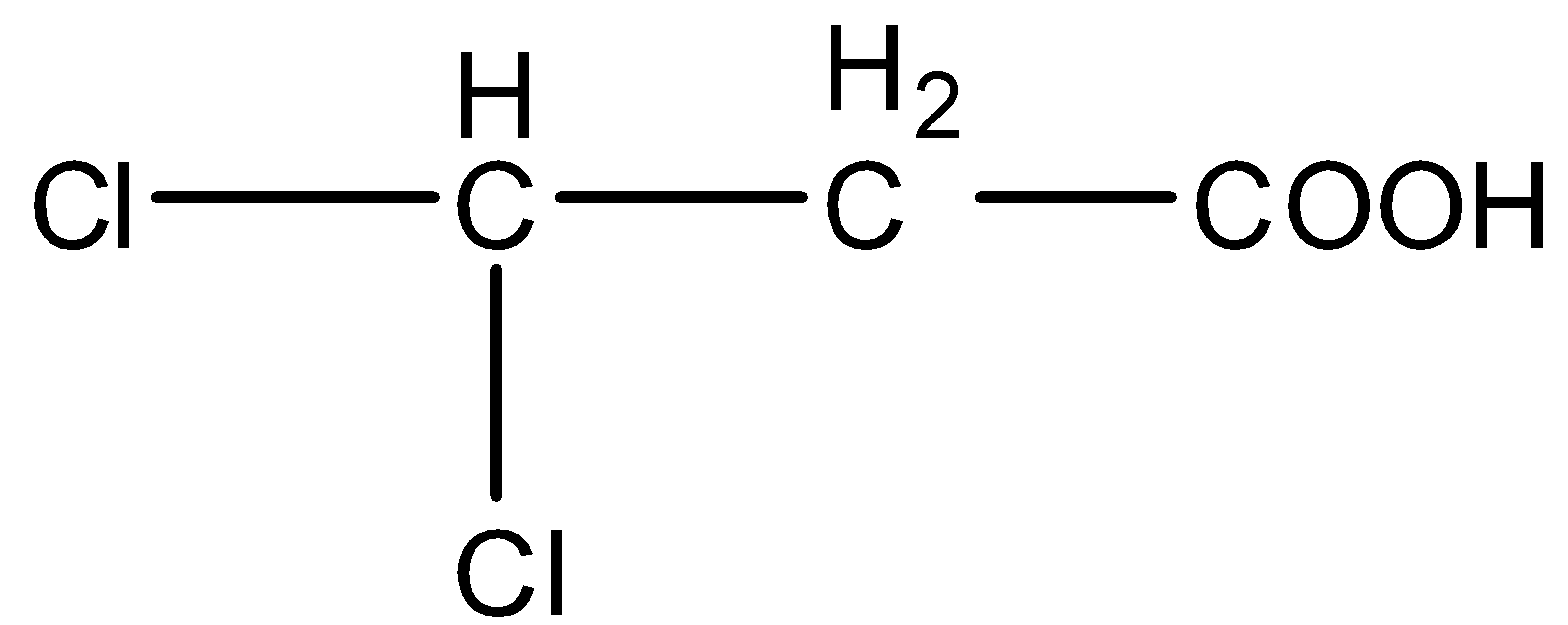
(B) 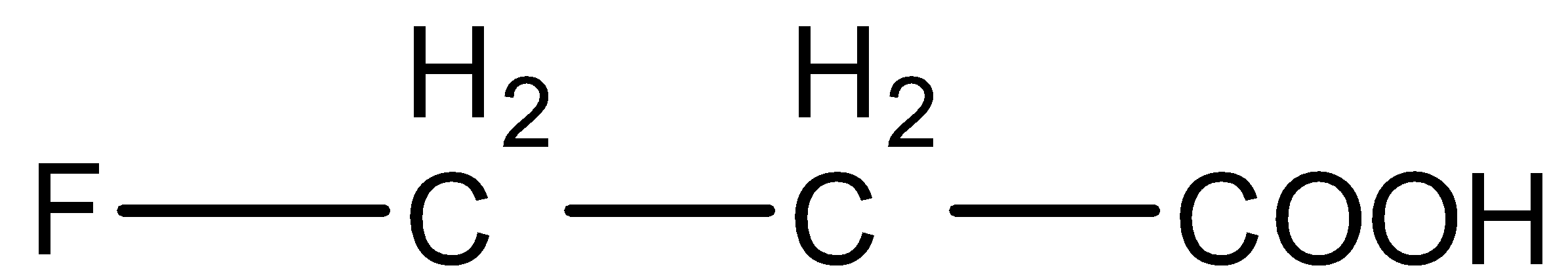
(C) 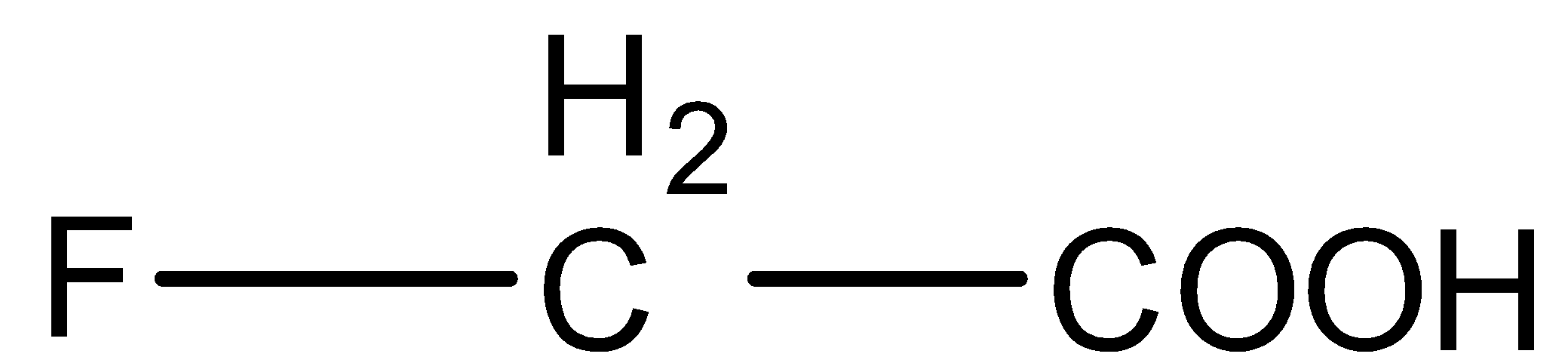
(D) 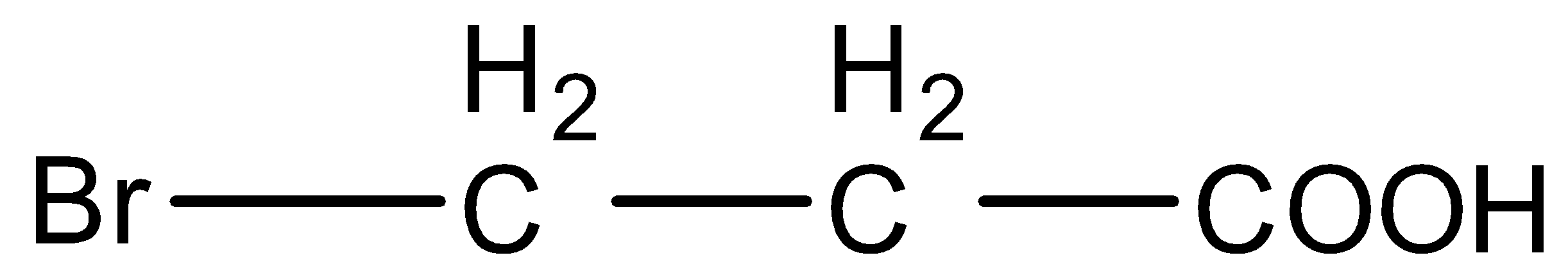
Solution
pKb is known as the dissociation constant of the base. pKb and pKa are related to each other by the equation pKa+pKb=14 . The strength of the acid depends upon the stability of the conjugate base.
Complete step by step answer:
First of all, let’s know about pKb.
- pKb is the negative logarithm of Kb which is the dissociation constant for the base. Thus, we can say that pKb=−log[Kb].
- Now, the compounds given to us are acids. So, we will first compare the strength of their acidity which will give the relation between their pKa and then we will find the relations between their pKb values which is asked in the question.
- We know that strength of an acid is determined by the stability of the conjugate base of that acid which is carboxylate ions. We can write that in form of reaction as
R−COOHH2OR−COO−+H+
- Thus, we can say that the more stable the carboxylate ion, the more stable the acid will be. We are given four of such acids. Let’s compare them.
- In option (C) fluorine atom is on α-carbon. None of the other acids given in the options have this. So, fluorine being the highest electronegative element will stabilize the negative charge on the oxygen atom and so the conjugate base will be stabilized. Thus, acid in option (C) is the most acidic.
- In option (A), there are two chlorine atoms. So, they being halogens will decrease the electron density on the negatively charged oxygen atom and stabilize the conjugate base. So, this will be the second most acidic compound from the given ones.
- In option (B) and (D), the only difference is the fluorine and bromine atoms. Fluorine is a more electronegative atom and hence it will stabilize the conjugate base more than bromine. So, Acid in option (B) will be more acidic than shown in option (D).
So, we can arrange the acids in the order of their acidic strength as (D) < (B) < (A) < (C).
- Now, we know that lower thepKa value, stronger the acid and higher the pKa value, weaker the acid. So, decreasing order of pKa values of these acids will be (D) > (B) > (A) > (C).
Now, we are being asked to compare their pKb values. We know that pKa and pKb are related by the following formula.
pKa+pKb=14
So, we can say that higher the pKa value of an acid, lower will be its pKb value.
So, we can arrange the acids given in the options in decreasing order of their pKb values as
(C) > (A) > (B) > (D).
Note: Remember that the effect of the substituent groups is most effective if the substitution is at α-position. If the substituent group is separated by more number of bonds with the carboxylic acid, its effect on the acidity of the acid decreases gradually.
