Question
Question: Arrange according to the order of stability. \(\text{ 3}{{\text{d}}^{\text{5}}}\text{ }\) , \(\tex...
Arrange according to the order of stability.
3d5 , 4d5 , 3d10 , 4d10
Solution
The stability of orbitals depends on the number of electrons present in the orbitals. There are two possibilities for orbitals to attain stability. Half-filled orbitals and full filled orbitals are stable as compared to any other arrangement of an electron in the orbitals. We are also well familiar with that the stability of the orbital or elements is inversely related to the energy. Higher energy less is stability.
Stability∝Energy1
Complete step by step answer:
An atom has a different shell, subshell, and orbitals. Electrons in the atoms are well distributed among this shell. All elements lean towards stability. The stability of an orbital in an element depends on the electrons in the orbital.
Orbitals attain stability if orbitals are:
- Filled orbitals:
Filled orbitals have filled orbitals. For example, the s orbitals contain the two electrons, p orbitals contain the 6 electrons, d subshell holds on 10 electrons, and f orbitals on 14. This is the complete filled orbitals, where each suborbital contains two electrons int. As they are filled, they do not undergo the loss or gain of electrons and thus elements with the filled orbitals are unreactive. For example, noble gases.
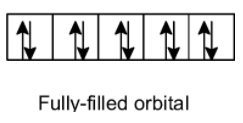
- Half-filled orbitals:
Half-filled orbitals in which each sub orbitals contains a single electron. For example, p orbitals contain the 3 electrons, d will hold on 5 electrons, and f will contain the 7 electrons. The half-filled orbitals are stable because here each orbital contains a single electron and thus symmetric. Do not readily react easily.
We have to find out the order of stability of the orbitals.
According to the Aufbau principle, the electron is filled in the atomic orbitals such that the lower orbital is filled first followed by the higher energy orbitals. According to the Aufbau energy trend diagram, the 3d orbitals are lower energy followed by the 4d orbital. We know that,
Stability∝Energy1
Therefore, 3d have more stability than the 4d orbitals.
Full filled orbitals are more stable than half-filled orbitals.
The order of stability of the orbitals is maximum for 3 orbitals and then for 4 orbitals. The order is given as follows,
4d5 < 4d10 < 3d5 < 3d10 .
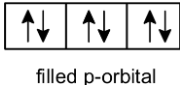
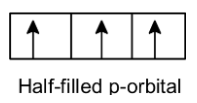
Note: The stability of full filled and half-filled orbitals is explained based on the total angular spin momentum of an orbital. Let’s take an example of p orbitals.
A) Half-filled p –orbitals:
p orbitals Have three electrons (let's consider all orbitals have parallel spins) then it is not completely stable.
B) Full-filled p-orbitals: p orbitals contained the 6 electrons, which have the opposite spin. Positive spins cancel out the negative spin and thus net spin is zero and thus fulfilled orbitals are more stable.