Question
Question: Aromatic compounds are: A)Open chain compounds B)Closed chain compounds C)Both open and closed...
Aromatic compounds are:
A)Open chain compounds
B)Closed chain compounds
C)Both open and closed chain compounds.
D)Closed chain compounds which are structurally similar to benzene.
Solution
We know that aromaticity is defined as the planar, cyclic molecule with the presence of resonance that provides stability to the molecule. Aromatic compounds are also known as conjugated planar ring systems. As here ring word is used so it cannot be open-chain structure
Complete step by step answer:
We must know that the aromatic compounds are also known as arenes.
We know that aromatic hydrocarbons contain sigma bonds and delocalized pi electrons between carbon atoms in the ring. Aromatic hydrocarbons are known as aromatic due to their pleasant smell.
We know that the best example of aromatic compounds is benzene:
Let us see the structure of benzene:
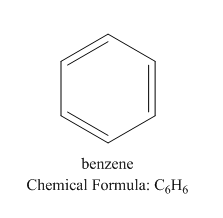
Here the pi bond inside the ring is responsible for the resonance and hence the stability of the compound.
Therefore, we can conclude that the correct answer to this question is option D.
Additional information: We know that hydrocarbon can be classified as an aromatic compound if they follow the Huckel rule. The Huckel rule contains three conditions for the molecules to be aromatic. Let us understand all three conditions with the help of a benzene molecule. The first condition is that a molecule should be planar and we know that benzene is a planar molecule. The second condition is resonance should be present in the molecule and benzene has two resonating structures. The third condition is there should be the presence of (4n+2)π electrons in the ring and here is the number of rings and is an integer (0, 1, 2, 3_ _ _ _) and we know that benzene has 6 pi electrons so by substituting one in (4n+2)π we get (4(1)+2)=6 pi electrons.
Note: We may get confused that every closed ring compounds are aromatic compounds but along with the closed chain it must follow all the conditions of the Huckel rule as benzene follows to become an aromatic hydrocarbon.
