Question
Question: Are \[{N_2}\] and \({N_2}^ + \) paramagnetic or diamagnetic? Which one has the stronger bond?...
Are N2 and N2+ paramagnetic or diamagnetic? Which one has the stronger bond?
Solution
To solve this question we should know about magnetic behavior of the elements, about their Molecular Orbital Diagram. Here in this question we have to draw the molecular orbital diagram of N2 and N2+ by which we can find the number of unpaired electrons. Hence with help of it, we can classify their magnetic nature.
Complete answer:
Paramagnetic substances are weakly influenced by the external magnetic field. They contain unpaired electrons in their orbitals. Paramagnetic substances have permanent dipole moments but with removal of magnetic field, the substance starts losing its magnetism.
Whereas, diamagnetic substances are those which do not get affected by external magnetic fields. They contain paired electrons in their orbitals. Diamagnetic substances show magnetization in the direction opposite to the magnetic field.
To find the magnetic behavior of the compound N2 , we have to draw the molecular orbital diagram of N2 by which we can get the number of unpaired electrons or paired electrons. As we know if a compound has unpaired electrons then that compound is paramagnetic in nature where if it contains paired electrons only then it is diamagnetic in nature.
The molecular orbital diagram of N2 :
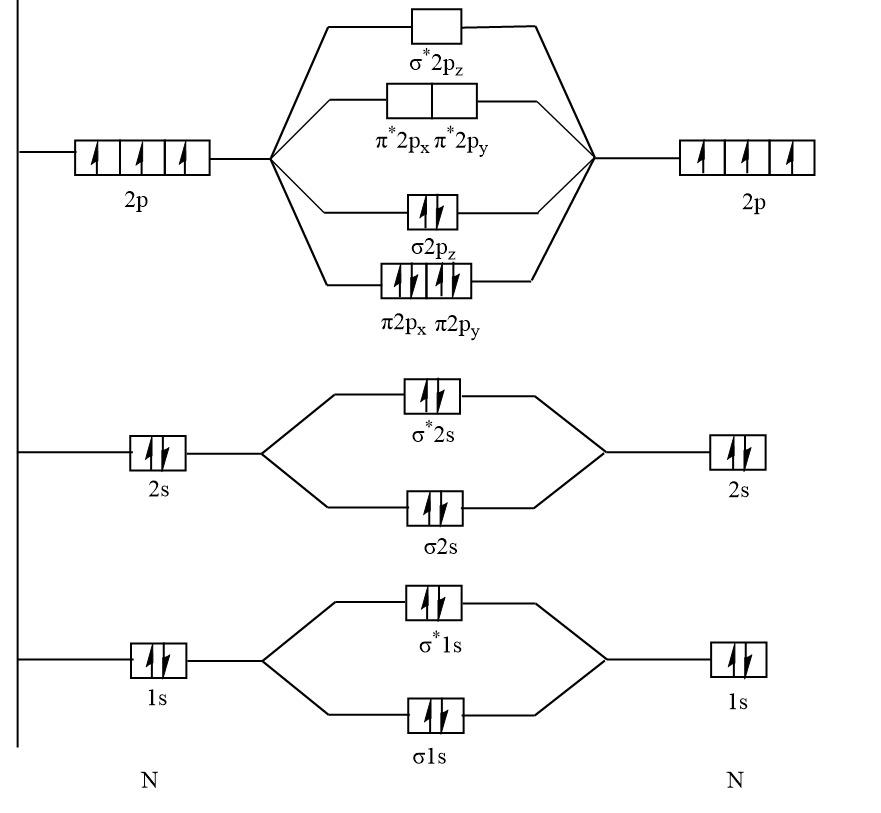
Here, with the help of a diagram it is clear that N2 has no unpaired electrons that means N2 is diamagnetic.
Again, to find the magnetic behavior of the compound N2+ , we have to draw the molecular orbital diagram of N2+ by which we can get a number of unpaired electrons or paired electrons.
The molecular orbital diagram of N2+ :
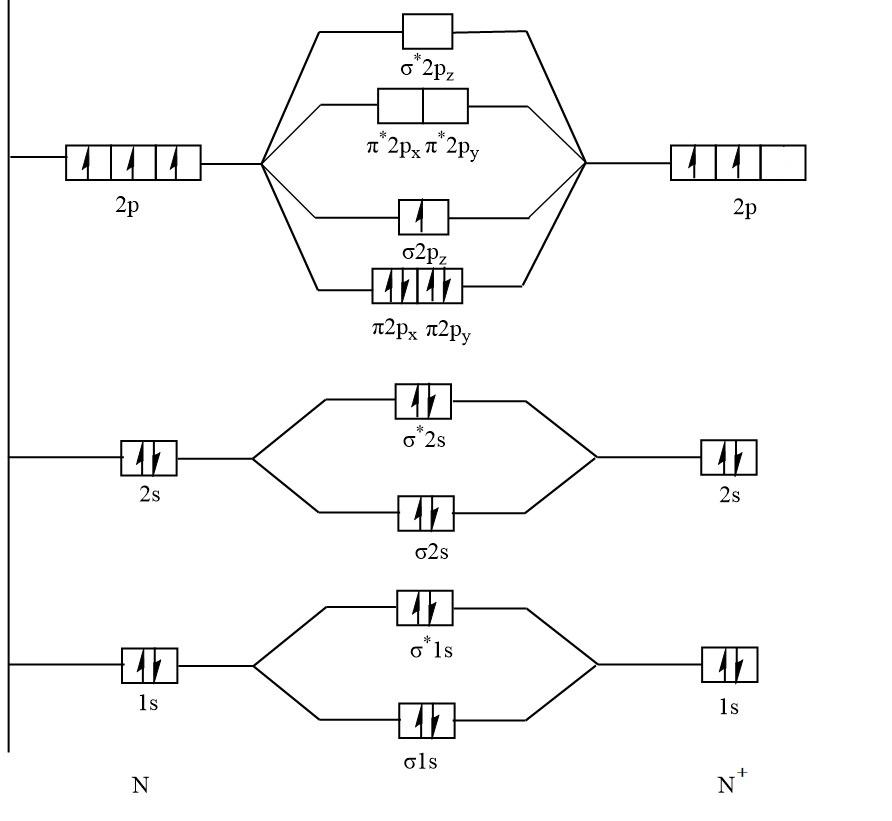
Again, with the help of a diagram it is clear that N2+ has unpaired electrons in σ2pz that means N2+ is paramagnetic.
Further, we know that after losing an electron in a bonding molecular orbital; the bond becomes less strong as it is less thermodynamically stable hence, N2+ has less bonding character than N2.
Note:
Remember compounds having electrons less than or equal to 14 have negligible s−p mixing and hence they follow normal pattern whereas compounds having more the 14 electrons have s−p mixing, leads to the pattern in which σp orbital is raised above the πp set.
