Question
Question: Are ethanol and diethyl ether the same molecule?...
Are ethanol and diethyl ether the same molecule?
Solution
A functional group is a substituent or moiety in a molecule that generates the molecule's distinctive chemical reactions in organic chemistry. Regardless of the remainder of the molecule's makeup, the same functional group will experience the same or comparable chemical processes. This allows for the systematic prediction of chemical reactions and compound behaviour, as well as the design of chemical synthesis. Other functional groups nearby can influence a functional group's responsiveness. Retrosynthetic analysis can be used to design organic synthesis via functional group interconversion.
Complete answer:
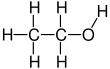
Ethanol is an organic chemical molecule that is also known as ethyl alcohol, grain alcohol, drinking alcohol, or just alcohol. C2H6O is the chemical formula for this simple alcohol. Its formula is CH3-CH2-OH !! !! or !! !! C2H5OH (an ethyl group joined to a hydroxyl group), and it is commonly abbreviated as EtOH. Ethanol is a colourless, volatile, flammable liquid with a distinctive odour. It is a psychotropic stimulant, as well as a recreational substance and a component in alcoholic beverages.
Diethyl ether, often known as Et2O, is a chemical compound in the ether class having the formula (C2H5)2O. It's a colourless, highly flammable liquid with a pleasant odour ("Ethereal odour"). It's frequently used in labs as a solvent and as a starting fluid in some engines. It was once employed as a general anaesthetic until non-flammable medicines like halothane were discovered. It has been used to induce drunkenness as a recreational drug. It's a butanol structural isomer.
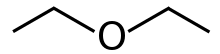
Hence they are not the same
Only ethanol and dimethyl ether are functional isomers.
The molecular formula of functional isomers is the same, but the functional group is different. As a result, dimethyl ether and ethyl alcohol are functional isomers.
Note:
The phenomenon of isomerism occurs when two or more compounds have the same chemical formula but distinct chemical structures. Isomers are chemical compounds with similar chemical formulas but differ in characteristics and atom arrangement in the molecule. As a result, substances that display isomers are referred to as isomers. The Greek words "isos'' and "meros," which indicate "equal portions," are used to create the term "isomer." In the year 1830, the Swedish scientist Jacob Berzelius invented the phrase.
