Question
Question: Aqueous sulphuric acid reacts with \( 2 \) -methyl- \( 1 \) -butene to give predominantly (A) Isob...
Aqueous sulphuric acid reacts with 2 -methyl- 1 -butene to give predominantly
(A) Isobutyl hydrogen sulphate
(B) 2 -methyl- 2 butanol
(C) 2 -methyl- 1 butanol
(D) Secondary butyl hydrogen sulphate
Solution
Alkenes are unsaturated compounds consisting of a double bond between the carbon and carbon atoms. Aqueous sulphuric acid can be used as a dehydrating agent. Thus, alkenes on treatment with sulphuric acid in presence of water undergo Markovnikov rule to form hydroxyl compounds.
Complete answer:
Alcohols are the compounds that consist of hydroxyl groups. Alkenes are unsaturated compounds consisting of a double bond between the carbon and carbon atoms.
Alkenes in presence of aqueous sulphuric acid forms alcohols. Alcohols are also known as hydroxyl compounds. Aqueous sulphuric acid can be used as a hydrating agent.
In this reaction, the water molecule adds to the alkenes, according to the Markovnikov rule. It states that the negative part of the water molecule which is hydroxide ion adds to the carbon with less number of hydrogen atoms. Whereas the positive part of the water molecule which is a proton, adds to the carbon with more hydrogen atoms.
The addition of aqueous sulphuric acid reacts to 2 -methyl- 1 -butene will be as follows:
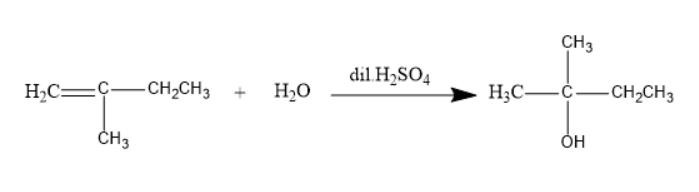
The product formed is 2 -methyl- 2 butanol.
Option B is the correct one.
Note:
Addition of alkenes takes place in two ways. One way is the Markovnikov rule and another way is anti- markovnikov rule. Anti- markovnicov rule takes place in presence of peroxides like hydrogen peroxide. In the given reaction there is no peroxide and it takes place according to the Markovnikov rule.
