Question
Question: Aqueous solution of sodium salt of 2-methylbutanoic acid on Kolbe electrolysis yields **P** (major p...
Aqueous solution of sodium salt of 2-methylbutanoic acid on Kolbe electrolysis yields P (major product). P is
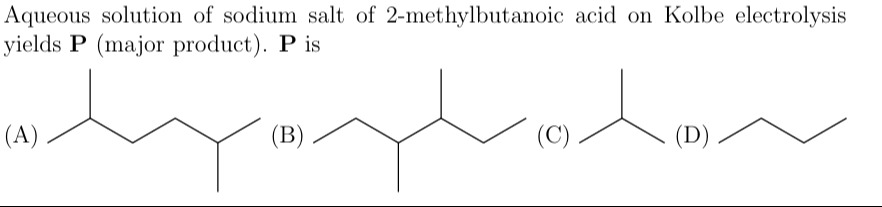
2-methylhexane
3,4-dimethylhexane
2-methylpropane (isobutane)
Butane
3,4-dimethylhexane
Solution
Kolbe electrolysis is a reaction that converts the sodium (or potassium) salt of a carboxylic acid into an alkane. The general mechanism involves the oxidative decarboxylation of the carboxylate ion at the anode, followed by radical dimerization.
1. Identify the starting material: The starting material is the sodium salt of 2-methylbutanoic acid.
2. Determine the alkyl radical (R•): In Kolbe electrolysis, the carboxylate ion (R−COO−) loses an electron to form an acyloxy radical (R−COO∙), which then decarboxylates to form an alkyl radical (R∙) and carbon dioxide (CO2). The R group in this case is:
CH₃
|
CH₃ - CH₂ - CH -
So, the alkyl radical formed is:
CH₃
|
CH₃ - CH₂ - CH• (This is a sec-butyl radical or 1-methylpropyl radical)
3. Dimerization to form the major product (P): Two identical alkyl radicals combine to form a symmetrical alkane. 2R∙→R−R Therefore, two sec-butyl radicals will combine:
CH₃ CH₃
| |
CH₃ - CH₂ - CH• + •CH - CH₂ - CH₃
This dimerization yields the product P:
CH₃ CH₃
| |
CH₃ - CH₂ - CH - CH - CH₂ - CH₃
4. Name the product P: To name the product, find the longest continuous carbon chain and number it to give the lowest possible locants to the substituents. The longest chain has 6 carbons (hexane). The methyl groups are located at positions 3 and 4. Thus, the name of the product P is 3,4-dimethylhexane.
