Question
Question: Aqua-regia is a mixture of hydrochloric acid and sulphuric acid. (A) True (B) False...
Aqua-regia is a mixture of hydrochloric acid and sulphuric acid.
(A) True
(B) False
Explanation
Solution
Hint: The mixture of nitric acid and hydrochloric acid gives a product that can dissolve gold. This product is used in cleaning gold ornaments. It is also used in cleaning glassware in labs.
Complete step by step solution:
- Aqua-regia was discovered by Jabir Bin Hayyan around the year 800AD.
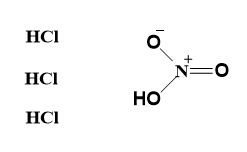
- Aqua- regia is actually a corrosive acid mixture of nitric acid (HNO3) and hydrochloric acid (HCl) in the molar ratio of 1:3.
- Aqua-regia is also known by the name- royal water. It is a yellow-orange coloured fuming liquid.
- Aqua-regia can be represented as-
- When the acids are mixed in the 3:1 ratio, the following reactions occur-