Question
Question: Applying the VSEPR theory, predict the shape of methyl isocyanate \(CH_3NCO\). The shape of \(CH_3NC...
Applying the VSEPR theory, predict the shape of methyl isocyanate CH3NCO. The shape of CH3NCO is predicted using valence shell electrons and Lewis dot structure.
Solution
In order to predict the geometry of molecules from a number of electron pairs surrounding their central atoms, we have a qualitative model known as Valence shell electron pair repulsion theory (VSEPR).
Complete answer:
The electron pair and lone pairs in valence shells around the central atom repel each other and tend to orient in space so as to maximize the repulsions and maximize the distance between them. Bond pairs shared by two toms and are attracted by two nuclei. Hence they occupy less space and cause less repulsion. On the other hand lone pairs are not involved in the formation of bonds, in attraction with one nucleus only therefore occupy more space and cause more repulsion Multiple bonds are treated as one single bond. The shape of molecules can be predicted from them from the number of electrons and type of electrons in the valence shell around the central atom. Double bond causes more repulsion than single bond.
Prediction of the structure of molecules with a single central atom is easy. Here in the question we have a molecule (CH3NCO) with no single central atom. We will start from carbon attached to 3 hydrogen and 1 nitrogen. One carbon is surrounded by 4 bond pairs. So this portion of molecule should have tetrahedral geometry just like CH4.
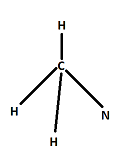
Now the nitrogen atom is bonded to other carbon by a double bond, therefore nitrogen is surrounded by 3 bond pairs, and then it must have one lone pair of electrons. Hence according to VSEPR theory, in this portion there should be a bent of 120∘, trigonal geometry.
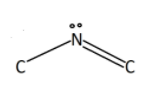
Now the next portion is carbon bonded to nitrogen and is also doubly bonded to oxygen atoms. It gives a total of 2 electron pairs to the carbon. Hence according to VSEPR theory, angle should be 180∘.
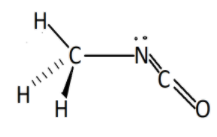
Hence the structure of methyl isocyanate will be :
Note:
Methyl isocyanate is a volatile and highly toxic molecule. In 1984, a large quantity of methyl isocyanate gas was accidently released when water leaked into storage tanks in Bhopal. The resulting highly exothermic reaction caused a rapid increase in pressure that ruptures the tanks. And they released a large amount of methyl isocyanate and killed almost 3800 people, disabled many.
