Question
Question: Answer the questions based on the diagram. The pressure at A and B in the atmosphere are respectiv...
Answer the questions based on the diagram.
The pressure at A and B in the atmosphere are respectively:
(A) 0.821 and 1.642
(B) 1.642 and 0.821
(C) 1 and 2
(D) 0.082 and 0.164
Solution
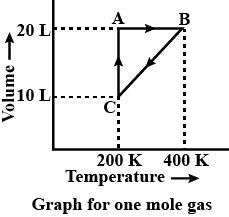
The given illustration is based on the equations of ideal gas laws. The given VT diagram is being evaluated for one mole of gas molecules present.
Complete Solution :
Let us first discuss the ideal gas law to properly answer the given question;
Ideal gas equation-
This is the empirical relationship between the volume of the gas, pressure of the gas, temperature of the gas and amount of the gas present. This can be represented as;
PV=nRT
where,
P = pressure
V = volume
n = amount of the substance
R = ideal gas constant
T = temperature
The ideal gas law is also called as general gas equation which is derived considering basic gas laws i.e. Boyle’s law and Charles law.
Now, considering this law, we solve illustration as:
At A-
Volume = 20 L
Temperature = 200 K
Pressure can be given as,
P=VnRTP=201×0.0821×200=0.821
So, pressure at A is 0.821 atm.
At B-
Volume = 20 L
Temperature = 400 K
Pressure can be similarly calculated as,
P=VnRTP=201×0.0821×400=1.642
So, the correct answer is “Option A”.
Note: Do note that here, nowhere is mentioned about the units of the quantities contributing the equation required to solve the given illustration. But we need to consider them properly by looking at the given data. As any mistake in the value of the ideal gas constant, It would result in chaos.
