Question
Question: Answer the questions based on diagrams. The process which occurs in going from, \(B\to C\) is: !...
Answer the questions based on diagrams.
The process which occurs in going from, B→C is:
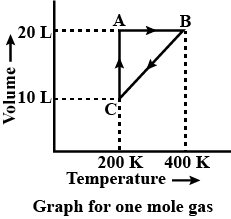
A. Isothermal
B. Adiabatic
C. Isobaric
D. Isochoric
Solution
While going from initial to final state in a thermodynamic state we have to go through several paths. State variables are defined only at that stage when the thermodynamic system is in equilibrium with the surrounding and this process is known as a quasi-static process.
Complete answer:
Thermodynamic process is generally of four types which are named as:
Isothermal, Adiabatic, Isobaric and Isochoric processes. To find out the answer of this question we have to discuss all the terms first.
A. Isothermal process: It is that thermodynamic process in which temperature remains constant. This process follows the first law of thermodynamics which showsQ=W.
B. Adiabatic process: In this process no heat is exchanged between the system and the surrounding. In this the value of heat transfer i.e. Q remains zero and work done is negative in this case.
C. Isobaric process: In this process pressure remains constant during the process. Graph given shows while going through the process from B→C volume and temperature is changing but the pressure is constant in this case.
D. Isochoric process: It is the process in which change in volume of the thermodynamic system is zero. This describes change in volume as zero so work done is also zero in this case.
Hence we can consider that option C is the correct answer.
Note:
Other than these four processes there is also one known process called cyclic process; it can be defined as the process in which the final state of the system is equal to the initial state. In easy manner we can say that change in internal energy is state function here i.e. ΔU=0.
