Question
Question: Answer the following questions for the below reaction:- \(Na\xrightarrow{{{H}_{2}}O}A\xrightarrow{...
Answer the following questions for the below reaction:-
NaH2OACO2BSO2CNa2S/I2ΔDAg+/saltE(complex)
1. The compound B and C are:-
(a) Na2CO3,Na2SO4
(b) NaHCO3,Na2SO4
(c) Na2CO3,Na2SO3
(d) None of these
2. The compound D is:-
(a) Na2SO4
(b) Na2S4O6
(c) Na2S2O5
(d)Na2S2O3
3. Oxidation no. of each ‘S’ atom in compound D:-
(a) +2, +2
(b) +4, 0
(c) +6,-2
(d) +5,-1
Solution
Attempt this question by solving the given reaction in steps. When a metal react with H2O, it produces a base. The base on further reaction with CO2 forms carbonate compound and H2O. The following product forms reacts with SO2 to give sulphide compounds. This sulphide compound when mixed with Na2S/I2, produces thiosulphate compound. After this calculate the oxidation state of ‘S’ atom in Thiosulphate compound by making its structure.
Complete step-by-step answer: Let’s discuss the chemical reactions given by the following compounds:-
-When sodium (metal) reacts with water, it gives Sodium Hydroxide and liberate hydrogen gas (that can be certainly checked by passing it through a beaker of soapy water which produces bubbles of H2 gas and when burning splinter is brought near it, it makes a pop sound).
Na+H2ONaOH+H2↑
-When sodium hydroxide reacts with carbon dioxide gas, it produces sodium carbonate along with water which is shown below:-
2NaOH+CO2Na2CO3+H2O
-When sulphur dioxide gas is passed through aqueous solution of sodium carbonate, displacement reaction takes place and the product formed is sodium sulphite with side products as carbon dioxide and water.
Na2CO3+H2O+SO2Na2SO3+CO2+H2O
-When iodine is added to the mixture of sodium sulphite and sodium sulphide, it produces sodium thiosulphate along with sodium iodide.
Na2SO3+Na2S/I2Na2S2O3+NaI
-When sodium thiosulphate is mixed with silver salt, it forms the following complex of silver:-
2Na2S2O3+Ag+/saltNa3[Ag(S2O3)2]+Na+salt
After discussing all these reactions we get to know that:-
A= NaOH
B= Na2CO3
C= Na2SO3
D= Na2S2O3
E= Na3[Ag(S2O3)2]
Now let’s come to the final part i.e., oxidation state of each ‘S’ atom in compound D =Na2S2O3
Oxidation state is the degree of oxidation of an atom in a compound or molecule or ion. It can be positive, negative or zero. We can calculate the oxidation state by drawing its structure as follows:-
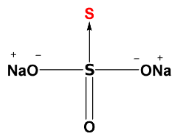
As we can see, there is a coordinate bond between 2 same atoms. Therefore the acceptor sulphur atom will have -2 oxidation state (highlighted by red color). Now, the other S atom i.e., the donor atom is surrounded by other groups as well, so we can calculate its oxidation state easily. The sum of the oxidation state of all the atoms will be equal to 0 (as the total charge on the compound is 0).
[Oxidation state of Na is +1 and O is-2]
Let’s consider x as the oxidation state of donor S atom, then:-
2(+1) + 3(-2) + x +1(-2) = 0
x = +6
So the oxidation state of acceptor S atom is -2 and donor S atom is +6.
Therefore the answers are as follows:-
1. (c) Na2CO3,Na2SO3
2. (d) Na2S2O3
3. (c) +6,-2
Note: Remember when SO2 react with dilute Na2CO3 , it produces NaHCO3. But this reaction takes place at 0∘C . So this would not be the product ‘C’. Also NaHCO3 does not give react with Na2S/I2. So make the product carefully.
-While calculating oxidation state, do not directly use “x” calculation method as followed:-
2(+1) + 3(-2) + 2x=0
x = +2
This oxidation state is not the degree of oxidation of each ‘S’ atom but it is the average oxidation state of the entire ‘S’ atom in the compound.
