Question
Question: Answer the following questions based on the P-T phase diagram of \(C{O_2}\). (A) \(C{O_2}\) at 1 a...
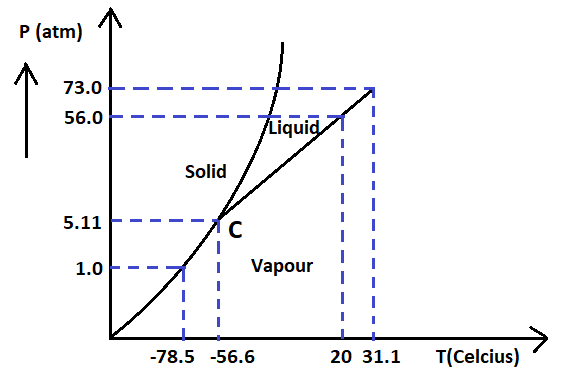
Answer the following questions based on the P-T phase diagram of CO2.
(A) CO2 at 1 atm pressure and temperature -60∘C is compressed isothermally. Does it go through a liquid phase?
(B) What happens when CO2 at 4 atm pressure is cooled from room temperature at constant pressure?
(C) Describe qualitatively the change in a given mass of solid CO2 at 10 atm pressure and temperature -60∘C as it is heated upto room temperature at constant pressure.
(D) CO2 is heated to a temperature 70∘C and compressed isothermally. What changes in its properties do you expect to observe?
Solution
To answer this question the graph must be studied carefully and necessary calculations should be made while solving the questions. The given graph is a pressure-temperature graph of a gas. The graph is also showing the different phases of the gas at different conditions of temperature and pressure.
Complete step by step answer:
Let’s look at the answer of the given question:
-In part (A) the gas is compressed isothermally at 1 atm pressure and -60∘C temperature. This temperature lies in the vapour phase of the gas. So, from the graph it is evident that the gas will get directly converted to solid.
Hence, it will not pass through the liquid phase.
-In part (B) the gas is cooled at 4 atm pressure which is less than 5.11 atm from room temperature. The gas will get directly converted into solid form without going through the liquid phase.
-In part (C) the gas is heated to room temperature from 10 atm pressure and -60∘C temperature. On heating the gas will first get converted to liquid and then to vapour form.
-In part (D) the gas is heated to 70∘C and compressed isothermally. 70∘C is much higher than the critical temperature, hence, no further liquefaction will occur. It will exist in vapour form only but will show more real gas behavior.
Note: Critical temperature of a gas is a temperature above which a gas cannot be liquefied however high pressure may be applied on the gas. A phase is a chemically and physically homogeneous part of a system. A substance can exist in all the three phases at the same temperature at a given pressure. This point is known as the Triple point of the substance.
