Question
Question: Answer the following – a. Give the resonating structure of a carboxylic group? b. Give chemical ...
Answer the following –
a. Give the resonating structure of a carboxylic group?
b. Give chemical reaction to obtain the following –
i. Carboxylic acid from Grignard reagent
ii. Benzoic acid from ethyl benzoate
iii. Benzamide from benzoic acid
c. Give resonating structures of propenoic acid?
d. How will you distinguish amongst aldehyde, ketone and carboxylic acid?
Solution
You can solve all these questions by understanding the basic difference amongst aldehydes, ketones and carboxylic acids. The basic structure of an aldehyde, ketone and carboxylic acid is –
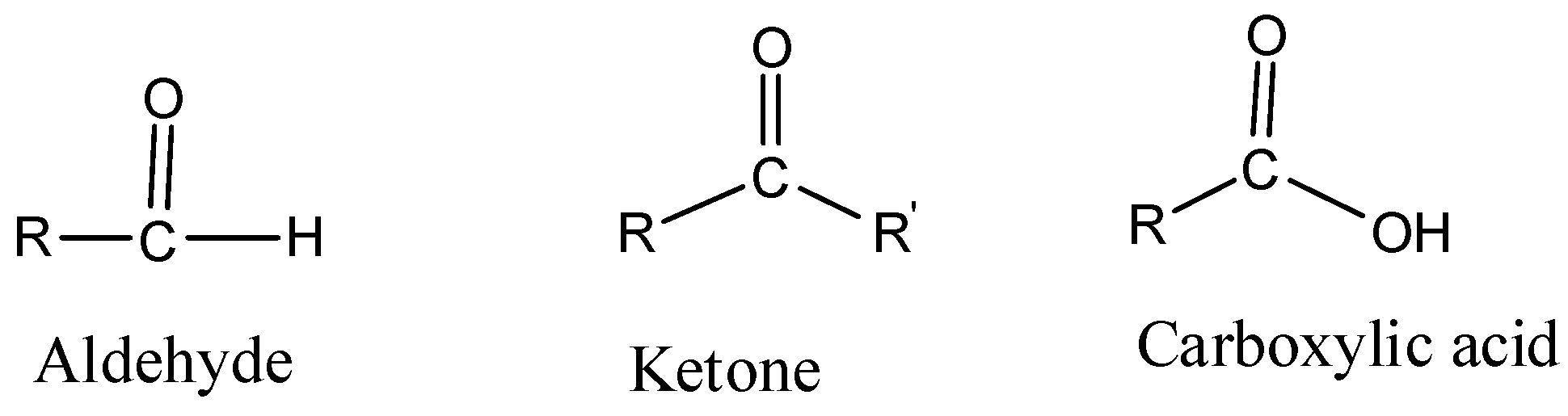
Complete step by step answer:
a.The resonating structures of carboxylic group are as given below –
- Let us begin by drawing the structure of a carboxylic acid.
- A carboxylic acid is given by a terminal carbon bonded to (-OH) group by a single bond and bonded to (-O) by a double bond.
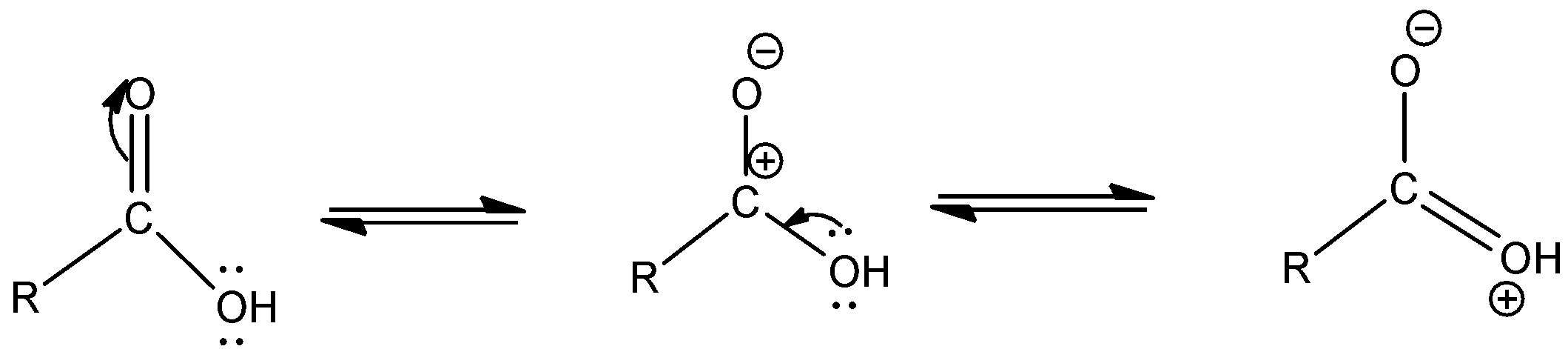
b. The chemical reactions are given below –
i. Carboxylic acid from Grignard reagent
- The general reaction for conversion of any compound to carboxylic acid is as given below –
R−MgX+CO2→RCOO−MgXH2OR−COOH+Mg(OH)X
ii. Benzoic acid from ethyl benzoate
- The reaction is as given below -
PhCOOEtNaOH−EtOHPhCOONaHCl−NaClPhCOOH
iii. Benzamide from benzoic acid
The reaction is as given below -
PhCOOH+NH3PhCOO−NH4+ΔPhCONH2+H2O
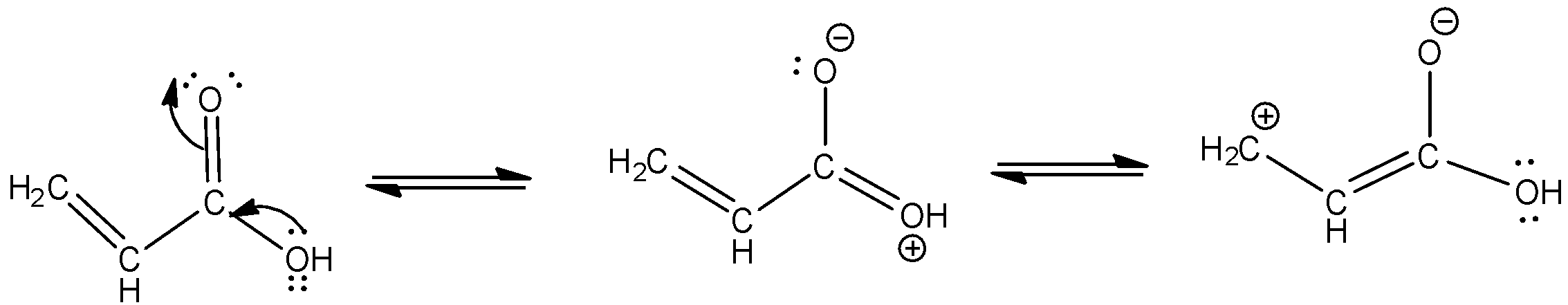
c. Resonating structures of propenoic acid –
- Let us begin by drawing the structure of a propenoic acid. As the name suggests, “prop-ene-oic acid” contains 3 carbons, 1 double bond and a carboxylic acid group.
-The structure and resonating structures are as given below -
d. We distinguish amongst aldehyde, ketone and carboxylic acid
- Aldehydes and Ketones can be distinguished by Tollen’s test. Whereas, carboxylic acids can be identified with sodium bicarbonate test, which gives effervescence of carbon dioxide gas upon reaction with sodium hydrogen carbonate.
- Tollen’s reagent is prepared by mixing silver nitrate with ammonium hydroxide. Aldehydes give a positive test – forms a silver mirror. Ketones give a negative test. Tollen’s reagent oxidizes aldehydes to corresponding acids and gets reduced to Tollen’s metallic silver.
Note: Note that aldehydes gives positive test for the silver mirror test or the Tollen’s test because aldehydes on treatment with the Tollen’s reagent is able to oxidise into the carboxylic acid that forms silver mirror.
