Question
Question: Answer Q.16, Q.17 and Q.18 by appropriately matching the information given in the three columns of t...
Answer Q.16, Q.17 and Q.18 by appropriately matching the information given in the three columns of the following table.
| Column I | Column II | Column III |
|---|---|---|
| (A) Only one organic product is formed | (P) Carbocation is formed as intermediate | |
| (B) Product is optically active | (Q) Carbanion is formed as intermediate | |
| (C) This process involves nucleophite substitution reaction at one of the carbon | (R) Free radical is formed as intermediate | |
| (D) This process involves electrophle substitioun reaction at one of the carbon | (S) Nitrene is formed as intermediate |
Which of the following option is correctly matched:
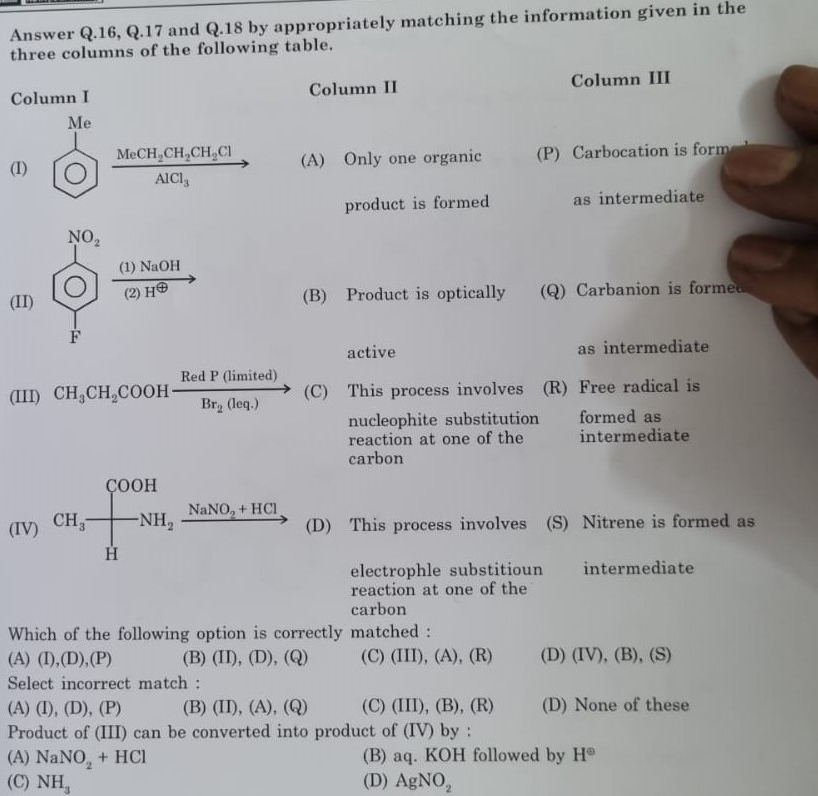
(I), (D), (P)
(II), (D), (Q)
(III), (A), (R)
(IV), (B), (S)
(A)
Solution
Let's analyze each reaction and match it with the properties and intermediates from Columns II and III.
Reaction (I): Friedel-Crafts alkylation of toluene with n-propyl chloride and AlCl3. This is an electrophilic aromatic substitution reaction. The electrophile is a carbocation formed from n-propyl chloride and AlCl3. Rearrangement of the initially formed primary carbocation (n-propyl) to a more stable secondary carbocation (isopropyl) occurs. The carbocation then attacks the activated aromatic ring (toluene) at ortho and para positions relative to the methyl group. Thus, a mixture of ortho- and para-n-propyltoluene (minor) and ortho- and para-isopropyltoluene (major) is formed. Column II: (A) Only one organic product is formed - Incorrect, multiple isomers are formed. (B) Product is optically active - Incorrect, the products are achiral. (C) Nucleophilic substitution - Incorrect. (D) Electrophilic substitution - Correct. Column III: (P) Carbocation is formed as intermediate - Correct. (Q) Carbanion - Incorrect. (R) Free radical - Incorrect. (S) Nitrene - Incorrect. Matching for (I): (D), (P).
Reaction (II): Nucleophilic aromatic substitution of 4-fluoro-1-nitrobenzene with NaOH. This is a nucleophilic aromatic substitution reaction via the addition-elimination mechanism (SNAr). The nucleophile (OH-) attacks the carbon bearing the leaving group (F), forming a resonance-stabilized carbanion intermediate (Meisenheimer complex). The leaving group (F-) is then eliminated. The product is 4-nitrophenol. Column II: (A) Only one organic product is formed - Correct (4-nitrophenol). (B) Product is optically active - Incorrect (4-nitrophenol is achiral). (C) Nucleophilic substitution - Correct. (D) Electrophilic substitution - Incorrect. Column III: (P) Carbocation - Incorrect. (Q) Carbanion is formed as intermediate - Correct (Meisenheimer complex). (R) Free radical - Incorrect. (S) Nitrene - Incorrect. Matching for (II): (A), (C), (Q).
Reaction (III): Hell-Volhard-Zelinsky (HVZ) reaction of propanoic acid with limited Red P and Br2. This reaction alpha-halogenates carboxylic acids. Propanoic acid has alpha-hydrogens. The product is 2-bromopropanoic acid. The alpha-carbon is chiral, so the product is chiral. The reaction typically proceeds via the formation of an acyl halide, enolization, and electrophilic attack by Br2 on the enol. A racemic mixture of (R)- and (S)-2-bromopropanoic acid is formed under standard conditions. Column II: (A) Only one organic product is formed - Incorrect (racemic mixture of enantiomers). (B) Product is optically active - Incorrect (racemic mixture is optically inactive). (C) Nucleophilic substitution - Incorrect. (D) Electrophilic substitution - Incorrect (substitution at alpha-carbon is not electrophilic aromatic substitution). Column III: (P) Carbocation is formed as intermediate - Possible during the reaction of the enol with Br2. (Q) Carbanion - Unlikely. (R) Free radical is formed as intermediate - Possible, although not the main pathway in standard mechanism. (S) Nitrene - Incorrect. Matching for (III): Based on the provided options, we need to find a consistent match. Let's re-examine the options for Q.16.
Reaction (IV): Reaction of alanine (an alpha-amino acid) with NaNO2 + HCl. This is the reaction of a primary amine with nitrous acid, forming a diazonium salt intermediate, which decomposes with the evolution of nitrogen gas. For alpha-amino acids, the decomposition typically involves the formation of a carbocation or free radical intermediate at the alpha-carbon, leading to substitution (usually by -OH from water) or elimination. The product is primarily 2-hydroxypropanoic acid (lactic acid). Since the starting material (alanine) is chiral and the reaction proceeds via a carbocation or free radical intermediate at the chiral center, racemization occurs, resulting in a racemic mixture of lactic acid. Column II: (A) Only one organic product is formed - Incorrect (racemic mixture of enantiomers, plus possibly alkene). (B) Product is optically active - Incorrect (racemic mixture is optically inactive). (C) Nucleophilic substitution - The overall reaction is a replacement of -NH2 by -OH, which is a substitution. However, the mechanism is complex. (D) Electrophilic substitution - Incorrect. Column III: (P) Carbocation is formed as intermediate - Correct (from diazonium ion decomposition). (Q) Carbanion - Incorrect. (R) Free radical is formed as intermediate - Possible (from diazonium ion decomposition). (S) Nitrene - Incorrect. Matching for (IV): (B) is incorrect. (P) or (R) is possible.
Let's check the provided options for Q.16 (correctly matched). (A) (I), (D), (P): (I) is (D) and (P). This is correct. (B) (II), (D), (Q): (II) is (A), (C), (Q). (D) is incorrect for (II). (C) (III), (A), (R): (III) product is a racemic mixture, so (A) is incorrect. (R) is a possible intermediate, but not the main one in the standard mechanism. (D) (IV), (B), (S): (IV) product is a racemic mixture, so (B) is incorrect. (S) is incorrect. So, option (A) is the only correctly matched option for Q.16.
