Question
Question: Anode rays were discovered by: (a). Goldstein (b). Crookes (c). Chadwick (d). Thomson...
Anode rays were discovered by:
(a). Goldstein
(b). Crookes
(c). Chadwick
(d). Thomson
Solution
- Hint: It was discovered by a German Scientist in 1886.
Complete step-by-step solution -
An anode ray (also positive ray or canal ray) is a beam of positive ions that is created by certain types of gas-discharge tubes. They were first observed in Crookes tubes during experiments by the German scientist Eugen Goldstein, in 1886.[1] Later work on anode rays by Wilhelm Wien and J. J. Thomson led to the development of mass spectrometry.
These rays are beams of particles moving in a direction opposite to the "cathode rays", which are streams of electrons which move toward the anode. Goldstein called these positive rays Kanalstrahlen, "channel rays" or "canal rays", because they were produced by the holes or channels in the cathode. In 1907 a study of how this "ray" was deflected in a magnetic field, revealed that the particles making up the ray were not all the same mass. The lightest ones, formed when there was some hydrogen gas in the tube, were calculated to be about 1840 times as massive as an electron. They were protons.
ADDITIONAL INFORMATION:
The process by which anode rays are formed in a gas-discharge anode ray tube is as follows. When the high voltage is applied to the tube, its electric field accelerates the small number of ions (electrically charged atoms) always present in the gas, created by natural processes such as radioactivity. These collide with atoms of the gas, knocking electrons off of them and creating more positive ions. These ions and electrons in turn strike more atoms, creating more positive ions in a chain reaction. The positive ions are all attracted to the negative cathode, and some pass through the holes in the cathode. These are the anode rays.
By the time they reach the cathode, the ions have been accelerated to a sufficient speed such that when they collide with other atoms or molecules in the gas they excite the species to a higher energy level. In returning to their former energy levels these atoms or molecules release the energy that they had gained. That energy is emitted as light. This light-producing process, called fluorescence, causes a glow in the region where the ions emerge from the cathode.
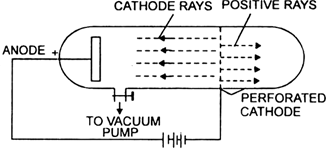
NOTE:
Properties of Anode rays are as follows:
They consist of positively charged particles.
They travel in straight lines and possess kinetic energy .
They are deflected towards electric and magnetic fields in the opposite direction to that of cathode rays.
They can penetrate thin metal foils.
They can ionise gas.
They produce mechanical effects.
