Question
Question: Aniline reacts with phosgene and excess of \( K\;OH \) to form : (A)  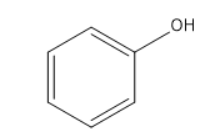
(B) 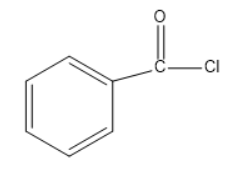
(C) 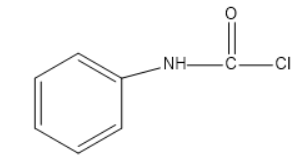
(D) 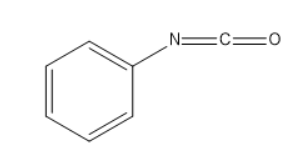
Solution
Hint : Try to solve this question by drawing the structure of phosgene and aniline in the presence of KOH taking into consideration the dipole moment, effect of presence of lone pair, polarity of bonds etc. This way you’ll be able to decipher the mechanism of the above reaction.
Complete Step By Step Answer:
Before the mechanism of the above reaction let us first familiarise ourselves with the structure of the compounds given in the question.
Aniline-
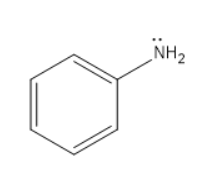
Aniline is the organic compound which consists of a phenyl group attached to an amino group. The amino group contains a lone pair which will participate in the above said chemical reaction.
Phosgene-
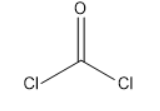
Mechanism:
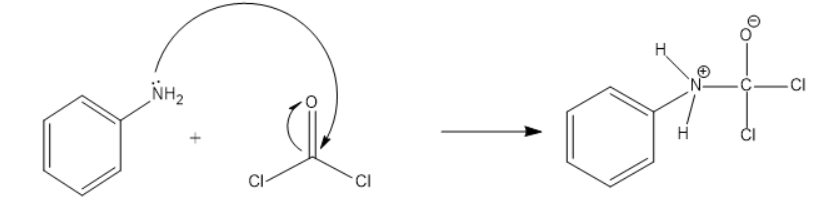
The lone pair on amine attacks the C of the phosgene and due to oxygen’s electronegativity the polarity moves towards the oxygen. The above product is formed. This negative charge on the oxygen will back off and will form a double bond which results in the release of Cl. This is shown below:
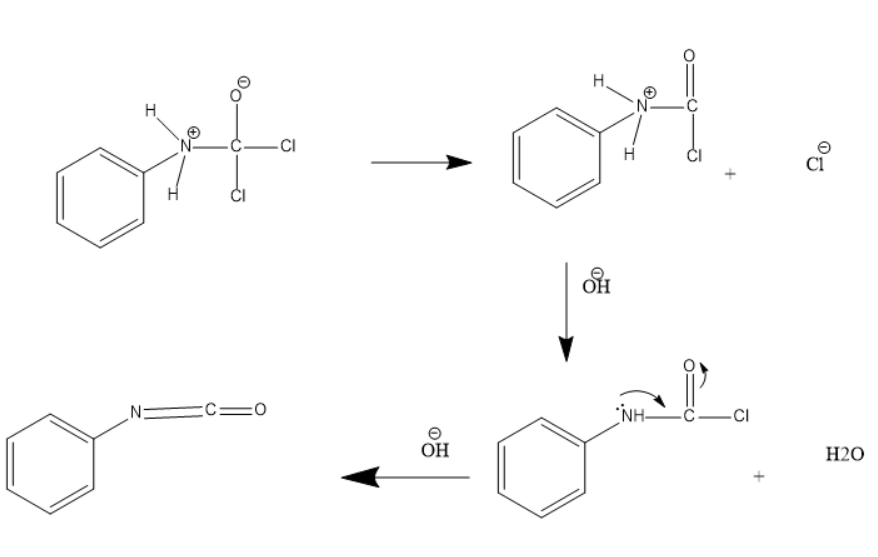
Then the ClOH− from potassium hydroxide attacks the above intermediate product which further results in release of water. On further reaction of the O{H^{-} ion which results in the formation of phenyl isocyanate.
The intermediate formed in the above reactions are unstable and therefore undergo rearrangement to achieve a stable structure.
**Therefore our answer is D.
Note :
Option a and b does occur in the above reaction but only as the intermediate not the final product. Also KOH can be written as K+OH− . The OH− in the above mechanism comes when the polarity of the potassium hydroxide is depicted individually.
