Question
Question: Aniline reacts with mixed acid (conc. HNO3 and conc. H2SO4) at 288 K to give P(51%), Q(47%) and R(2%...
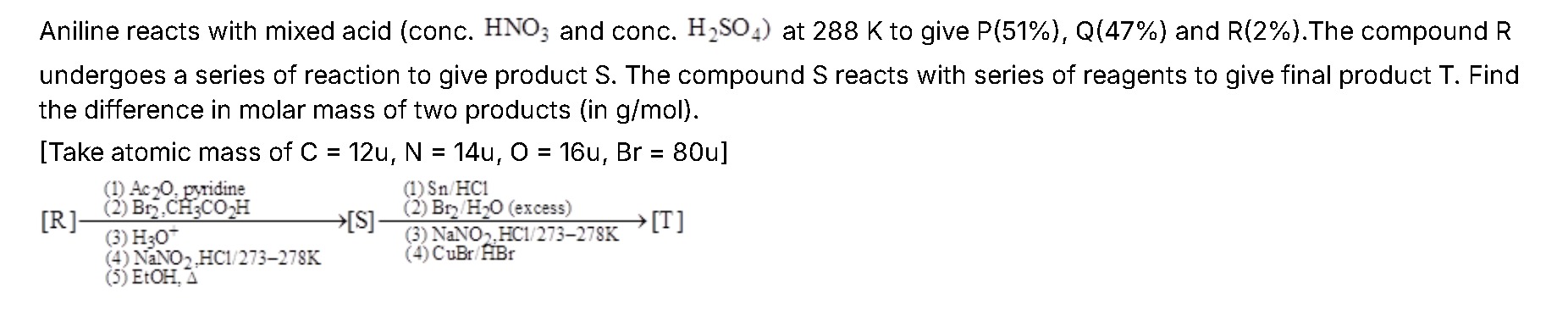
Aniline reacts with mixed acid (conc. HNO3 and conc. H2SO4) at 288 K to give P(51%), Q(47%) and R(2%). The compound R undergoes a series of reaction to give product S. The compound S reacts with series of reagents to give final product T. Find the difference in molar mass of two products (in g/mol).
[Take atomic mass of C = 12u, N = 14u, O = 16u, Br = 80u]
129 g/mol
Solution
Solution:
-
Identification of R:
Aniline nitration under mixed‐acid conditions gives mainly meta products, but the very minor product (R, 2%) comes from the unprotonated aniline and is the para‐nitration isomer, i.e. p‑nitroaniline. -
Conversion of R to S:
- (a) Ac₂O/pyridine converts the –NH₂ into an acetamide (p‑nitroacetanilide).
- (b) Bromination in CH₃CO₂H occurs ortho to the acetamido group; due to the para –NO₂, bromination takes place predominantly at the available ortho position (say at C–2).
- (c) Hydrolysis (H₃O⁺) reconverts the acyl to –NH₂ giving 2‑bromo‑p‑nitroaniline.
- (d) Diazotization (NaNO₂/HCl at 273–278 K) of the aniline followed by reaction with EtOH (Δ) replaces the diazonium group by –OEt.
Thus, S is 1‑ethoxy‑2‑bromo‑4‑nitrobenzene.
Its formula:
Benzene ring (C₆) + ethoxy (C₂H₅O) = C₈; substituents: Br and NO₂; remaining ring H’s = 3.
Molar mass = 8×12 + 8 + 80 + (14+32) = 96 + 8 + 80 + 46 = 230 g/mol. -
Conversion of S to T:
- (a) Sn/HCl reduces –NO₂ to –NH₂ giving 1‑ethoxy‑2‑bromo‑4‑aminobenzene.
- (b) Bromination with excess Br₂/H₂O causes electrophilic substitution directed by the –NH₂. With positions 2 (already Br) and 1 (ethoxy) occupied, bromination occurs at the available ortho position relative to –NH₂ (taken here as C–5) yielding a dibromo intermediate: 1‑ethoxy‑2,5‑dibromo‑4‑aminobenzene.
- (c) Diazotization (NaNO₂/HCl, 273–278 K) converts the –NH₂ to the diazonium salt which, on reaction with CuBr/HBr, is replaced by bromine.
Thus, T is 1‑ethoxy‑2,4,5‑tribromobenzene.
Its formula: Benzene ring with substituents: ethoxy (C₂H₅O), and three bromines; the ring retains 2 H’s.
Molar mass = 8×12 + 7 + 3×80 + 16 = 96 + 7 + 240 + 16 = 359 g/mol. -
Difference in molar masses:
Difference = 359 g/mol – 230 g/mol = 129 g/mol.
Minimal Explanation:
R (p‑nitroaniline, 2%) → acetylation → bromination (ortho to acetamide) → hydrolysis → diazotization/ethoxylation yields S (1‑ethoxy‑2‑bromo‑4‑nitrobenzene, 230 g/mol). Reduction of –NO₂ to –NH₂, bromination (introducing another Br at an activated ring position), and diazotization/CuBr replacement gives T (1‑ethoxy‑2,4,5‑tribromobenzene, 359 g/mol). Difference = 359 – 230 = 129 g/mol.
