Question
Question: Aniline is soluble in which of the following organic reagents? a.) Benzene b.) Ether c.) Alcoh...
Aniline is soluble in which of the following organic reagents?
a.) Benzene
b.) Ether
c.) Alcohol
d.) All of the above
Solution
Try to analyze the physical properties of the aniline. Solubility is also a physical property which says how ease a compound can dissolve or mix in another compound. Also discuss the nature of the groups that are attached to the benzene ring in the aniline molecule.
Complete Solution :
The molecular formula of the aniline is C6H5−NH2 which is having a typical structure as shown below
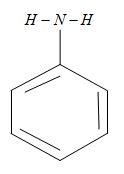
The polarity of this molecule is very low and also the intermolecular hydrogen bonding in itself is not that much strong so that it can form a hydrogen bonding with itself which makes it not to dissolve in any organic molecule. These properties make the aniline to solve in the organic molecules and it can also form the hydrogen bonding with the molecules of the other molecules other than the aniline. And hence aniline can dissolve in organic reagents like benzene, ether, alcohol. That means aniline can soluble in all the organic reagents given above.
So, the correct answer is “Option D”.
Note: In non polar solvents like CS2 the aniline will exhibit high resonance and due to this it becomes ready to undergo reduction. Due to this effect the ortho and the Para positions are substituted by reducing the NH2 group that is attached in the aniline and the process is called as the mono-substitution.
