Question
Question: ‘Anhydrone’ is a very effective desiccant (water absorber) used in ‘dry batteries’. It is: A. con...
‘Anhydrone’ is a very effective desiccant (water absorber) used in ‘dry batteries’. It is:
A. conc.H2SO4
B. P2O5
C. CaCl2
D. Mg(ClO4)2
Solution
Hint A dry cell is a portable electric battery that is used mainly in homes for flashlights, radios, remote controllers, and many other devices. It converts chemical energy into electrical energy like any other battery.
Complete step by step answer:
- A cell is defined as the single source of electrical energy which produces the energy by chemical reaction.
- A dry cell is a type of electrochemical cell which has electrolyte immobilized as a paste, with moisture enough to allow the flow of current.
- Dry cells can be used in any orientation as the cell doesn’t contain free liquid.
- Magnesium perchlorate is commonly known as Anhydrone. The chemical structure is shown below
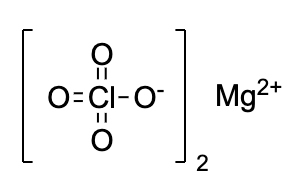
- Magnesium perchlorate appears as a white crystalline solid. It absorbs water from air and dissolves in it completely. And so, it is used as a regenerable drying agent.
- Magnesium dry cells don’t need to be stored in refrigerators like other conventional zinc dry batteries.
- Therefore, the answer to the question is option (B) Mg(ClO4)2
Note: The dry cell has electrolyte which is used to transfer the ions from anode to the cathode at the time of discharge of the cell. The ions move from the cathode to anode at the time of charging of the cell and the chemical reactions allow us to make this charging and discharging possible.
