Question
Question: An yellow coloured liquid (A) called as ‘Oil of Mirbane’ is reduced with \(Sn/HCl\) to give compound...
An yellow coloured liquid (A) called as ‘Oil of Mirbane’ is reduced with Sn/HCl to give compound (B). Identify A and B and write the equation.
Solution
Hint :First all we have to identify compound (A) and (B) to solve this reaction. There are some compounds in chemistry who are known by other names, oil of mirbane is also one of them. As it is yellow coloured liquid so this gives an hint to identify the compound.
Complete step by step:
Nitrobenzene is also known as ‘Oil of mirbane’. Its chemical formula is C6H5NO2. Nitrobenzene is a pale yellow to yellow brown liquid coloration in liquid form which is insoluble in water. It becomes a greenish yellow crystal when frozen.
So we have identified compound (A) that is nitrobenzene, now it is mentioned in the problem that nitrobenzene reduced with Sn/HCl to give compound (B). So in acidic medium with tin nitrobenzene reduces to aniline whose chemical formula is C6H5NH2. The reaction of nitrobenzene is reduced with Sn/HCl is as follows.
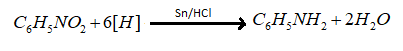
Thus compound (A) is nitro benzene and compound (B) is aniline, both are aromatic compounds. Hence we can say that (A) is nitrobenzene.
Additional information- The main use of nitrobenzene is to produce aniline approximately 95% of nitrobenzene is used in the production of aniline. It is also used in leather dressing, paint solvents, soaps, Kerr cells etc. It is used as a mild oxidant in reactions.
In aniline a nitro group is attached to a benzene ring. It is the simplest aromatic amine. Other names of aniline are phenylamine, aminobenzene, benzamine. It is produced by nitrobenzene at industry level. It is used in rubber processing, dyes and illustrative of the drug that is prepared from aniline is paracetamol etc.
Note : Hence we have solved the problem by identifying compound (A) that is nitrobenzene which is also known as ‘Oil of Mirbane’ a very poisonous, flammable pale yellow aromatic compound. When nitrobenzene reacts with Sn/HCl it reduces to aniline which is also an aromatic compound.
