Question
Question: An unsaturated hydrocarbon ‘A’ adds two molecules of \({H_2}\) and on reductive ozonolysis gives but...
An unsaturated hydrocarbon ‘A’ adds two molecules of H2 and on reductive ozonolysis gives butane-1,4-dial, ethanal and propanone. Give the IUPAC name of A.
(A) 3-methylocta-2,6-diene
(B) 2-methylocta-2,5-diene
(C) 2-methylocta-2,6-diene
(D) 2-methylocta-3,5-diene
Solution
Addition of H-atom on alkene is called hydrogenation. The addition of ozone to alkene molecules to form ozonide is called ozonolysis. This reaction is useful in detecting the position of double bond in alkenes. Use the properties that ‘A’ should have to go through hydrogenation and ozonolysis.
Complete Step by step answer: Hydrogenation and ozonolysis is done with alkene. So it is clear that it is an alkene.
Since during reduction of alkene, two moles of H2 molecules are required. Therefore, the alkene must contain two double bonds.
It means it is a diene.
It is given that, on ozonolysis, the alkene produces butane-1,4-dial, ethanol and propanone.
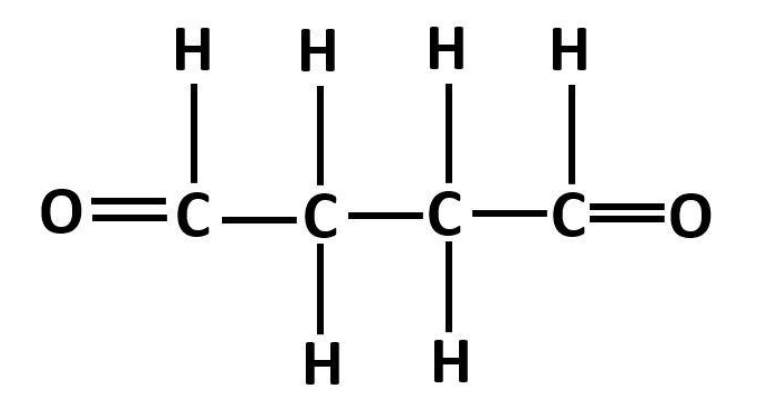
Structure of Butane-1,4-diol is
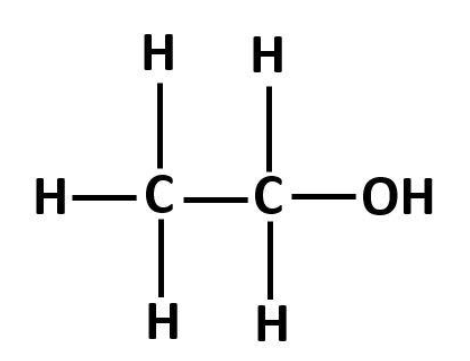
Structure of ethanol is
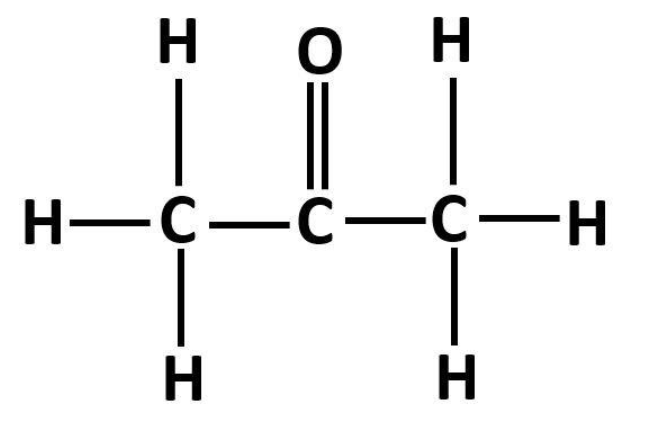
The structure of propanone is
We can arrange the above molecule as follows to identify the alkene.
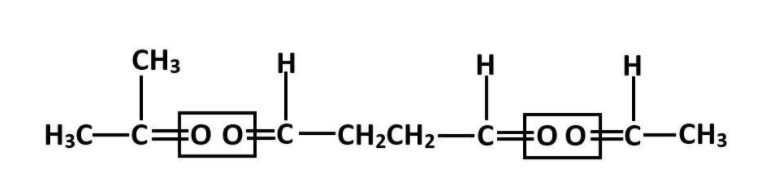
Now, these oxygen bonds are the result of ozonolysis on the alkene that we are trying to find. So we can reverse engineer it and assume that before ozonolysis, the oxygen molecules would not be there.
So, Remove O-atoms from adjacent molecules. Thus the following structure is formed.
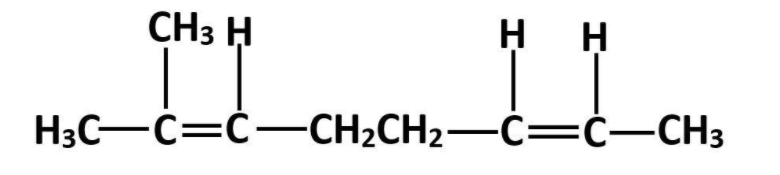
This is 2-methyl octa-2,6-diene.
Therefore, from the above explanation the correct option is (C) 2-methylocta-2,6-diene.
Note: We have solved this question by thinking in the reverse direction. This method helps in such cases, where we start thinking about the end and come towards the first step. Like in this question, we first considered the results of the reaction which were given, then we thought about the products they had before the reactions like ozonolysis were done one them. So, this example shows us that we should just mug up the chemical reactions but also understand the mechanism behind them.
