Question
Question: An unknown compound has a molecular formula \({{{C}}_{{6}}}{{{H}}_{{{10}}}}\). The unknown reacts wi...
An unknown compound has a molecular formula C6H10. The unknown reacts with H2in presence of Ptat high temperature to give C6H12. What information does it provide about the structure of the unknown?
(A) It has only one olefinic bond and a cyclic structure.
(B) It has two olefinic bonds and a cyclic structure.
(C) It has aromatic structure with two olefinic bonds.
Solution
First we make the structure of the compounds according to the specifications given in the option using six carbon chains only . By those structures we match the molecular formula of reactant and product with the molecular formula we want in the question above.
Complete step by step answer:
As said in the question that the compound react with H2and two atoms of hydrogen are added because C6H10 converts to C6H12 that clearly shows that one double bond or triple bond was present upon which the hydrogen added. Now let's check double or triple bonds.
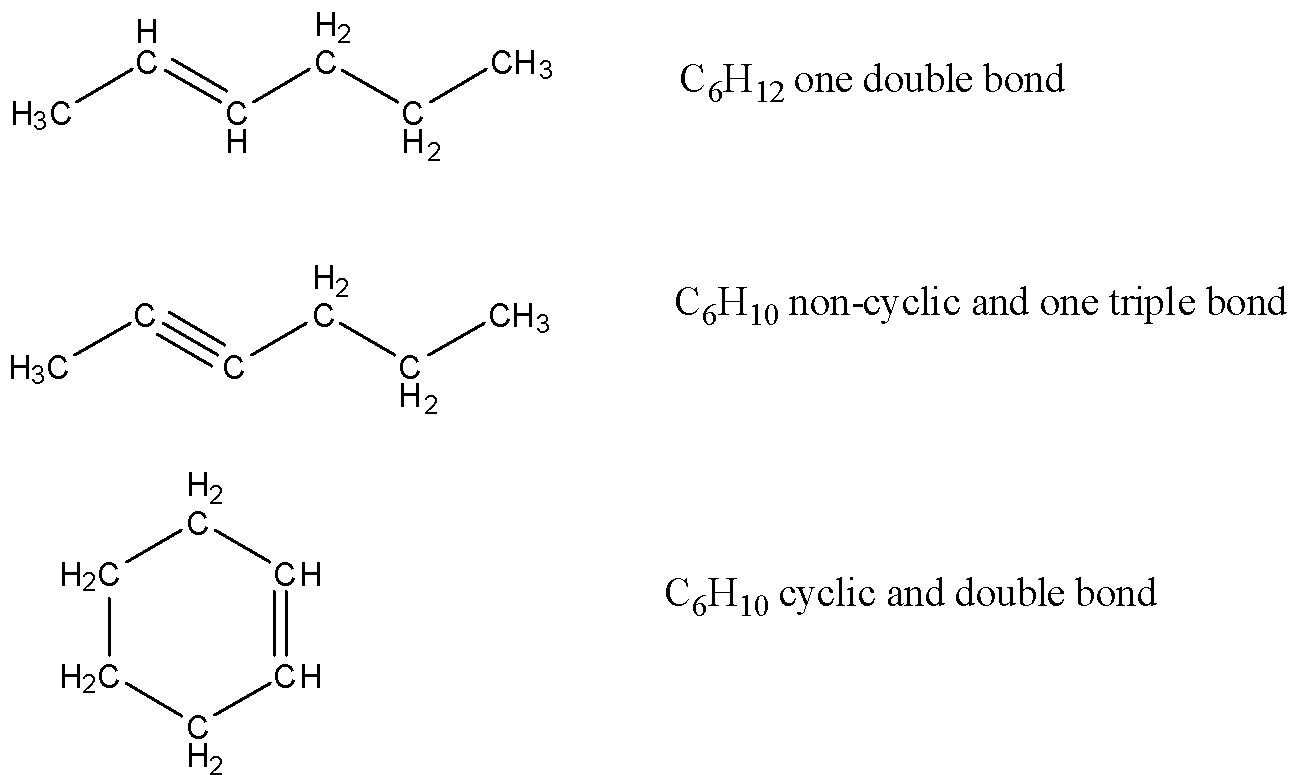
The above three structures have been labeled with a number of carbon and hydrogen. According to it the first structure is eliminated as there should be 10H , now second structure can be right but seeing the options in the answer it should be either cyclic or aromatic so this triple bond option is also eliminated and we are remained with last structure cyclohexene.
We cannot take aromatic compound as aromatic structure of six carbon will be benzene and its chemical formula is C6H6 which does not matches with the molecular formula given in the question.
It cannot have 2 olefinic bonds as the ratio of hydrogen and carbon will again not be similar to C6H10
So, the correct answer is Option A.
Additional information:
Cyclohexane is not a planar molecule, it is present in the form of a chair. But with cyclohexene due to steric hindrance and bond rigidity the chair form of cyclohexene converts to twisted chair form.
Note: Here is the structure of the twisted chair form
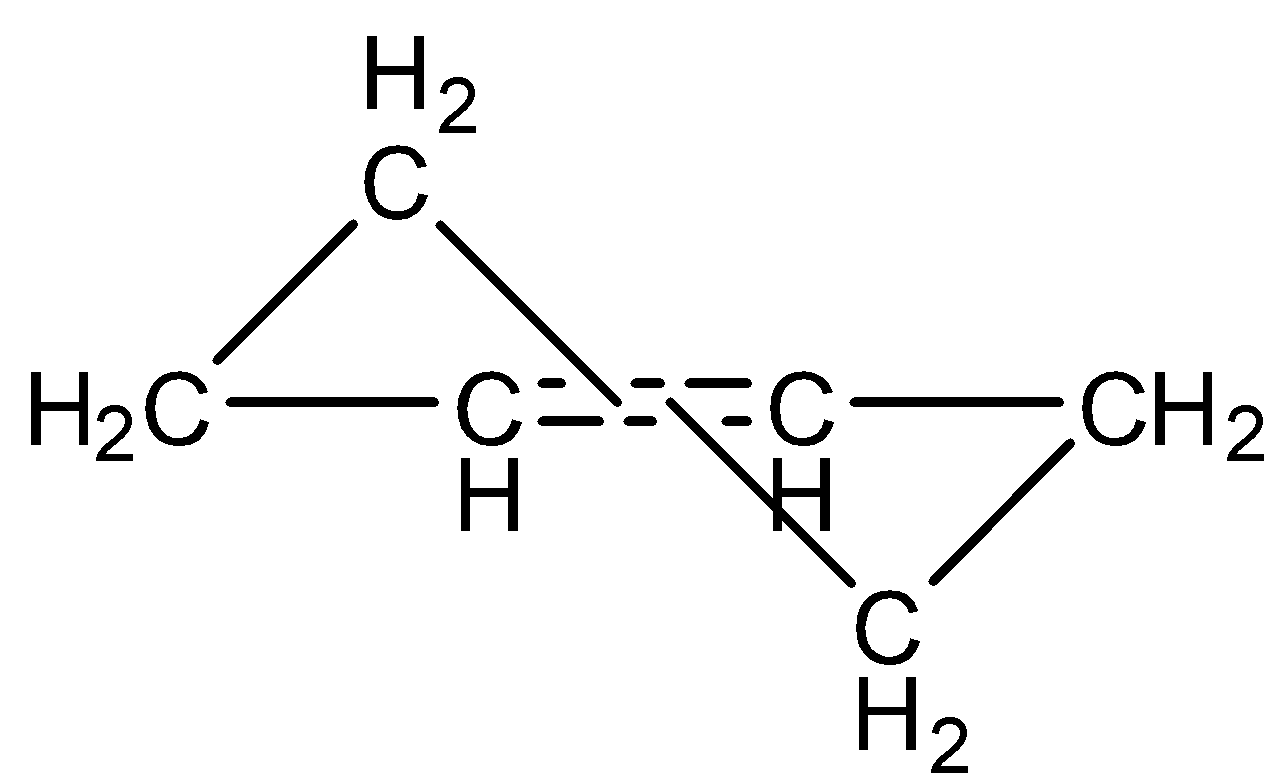
The attack of nucleophiles in twisted chair form is different. Nucleophile attack from above for one carbon in double bond and from below for the other carbon in the double bond.
