Question
Question: An unknown compound \[(A)\]has a molecular formula \[{C_4}{H_6}.\]When \[(A)\]is treated with excess...
An unknown compound (A)has a molecular formula C4H6.When (A)is treated with excess of Br2 a new substance(B) with formula C4H6Br4 is formed. (A) forms a white ppt. with ammoniacal silver nitrate solution.(A) may be:
A. But-1-yne
B. But-2-yne
C. But-1-ene
D. But-2-ene
Solution
C4H6 is alkyne. Alkynes are unsaturated hydrocarbons having triple bonds between at least two adjacent carbon atoms.
It’s general formula is CnH2n−2
Complete step by step answer:
Let us form an equation according to the given condition
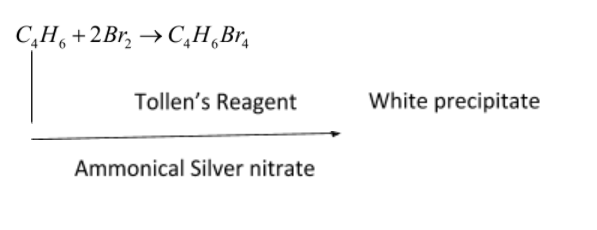
C4H6is butyne. It can either be But-1-yne or But-2-yne.
CH3−CH2−C≡CH But-1-yne
CH3−C≡C−CH3 But-2-yne
If the triple bond is present with the carbon atom 1 and 2, then the hydrogen atom is present at C-atom 1 so it is acidic in nature.
CH3−CH2−C≡CHis acidic in nature.
Since Ammoniacal silver nitrate is reducing agent, it reduces butyne, and butyne oxidize ammonical silver nitrate solution.
We know that oxidation and Reduction occur together.
But-1-yne reduces by tollen’s reagent and form white precipitate
CH3−CH2−C≡C−H+
So, the correct answer is “Option A”.
Additional Information:
Tollen’s Reagent is an ammoniacal solution of AgNO3. It is prepared by adding NH4OH solution toAgNO3 solution till the precipitate of Ag2O is formed which just gets dissolved. It has the formula [Ag(NO3)2]+OH−
2AgNO3+2NH4OH→Ag2O+2NH4NO3+H2O
Ag2O+4NH4OH→2[Ag(NH3)2]+OH−+H2O
We can define oxidation and reduction as follows:
Addition of oxygen or removal of hydrogen is called Oxidation.
Addition of hydrogen or removal of oxygen is called Reduction.
Since But-1-yne is acidic in nature. So it can easily remove H-atoms and oxidates, while Tollen’s reagent reduces. But-2-yne does not have acidic hydrogen, so it will not react with tollen’s reagent.
Note:
Oxidation and reduction occurs simultaneously. This reaction is a Redox reaction in which But-1-yne reduces and Tollen’s reagent oxidises.
