Question
Question: An uncatalysed reaction’s progress line is shown by the solid line. Which of the following dotted li...
An uncatalysed reaction’s progress line is shown by the solid line. Which of the following dotted lines represent the same reaction in the presence of a catalyst.
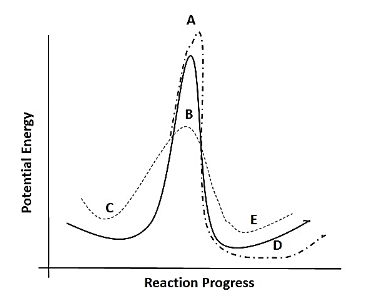
A. A
B. B
C. C
D. D
E. E
Solution
A catalyst is defined as a chemical or biological substance that helps to enhance the rate of reaction between the reactants without itself undergoing any change in its concentration. It decreases the activation energy required for the reaction.
Complete step-by-step answer: The minimum amount of energy that is required for the reaction to take place so that the bonds among the reactant molecules are broken and new bonds are formed is called the activation energy. Often this activation energy is so high that either it takes a high amount of energy for the reaction to take place or the reaction does not take place at all. To lower this activation energy of the reaction, the catalyst is used which provides and alternate route for the reaction and thus lowers the activation energy. The activation energy is mainly dependent upon the efficiency of the collisions between the molecules and a catalyst helps to increase the rate of the reaction between the molecules.
Thus, keeping these points the dotted line that represents the rate of the reaction in presence of the catalyst is B, hence the correct option is B.
Note: The catalyst however does not have any influence on the equilibrium of the reaction as it favours the rate of both the backward as well as the forward reaction so ultimately the equilibrium remains the same.
