Question
Question: An oxygen cylinder of volume 30 litre has 18.20 moles of oxygen. After some oxygen is withdrawn from...
An oxygen cylinder of volume 30 litre has 18.20 moles of oxygen. After some oxygen is withdrawn from the cylinder, its gauge pressure drops to 11 atmospheric pressure at temperature 27°C. The mass of the oxygen withdrawn from the cylinder is nearly equal to:
[Given, R=12100Jmol−1K−1, and molecular mass of O2=32, 1 atm pressure = 1.01×105N/m2]
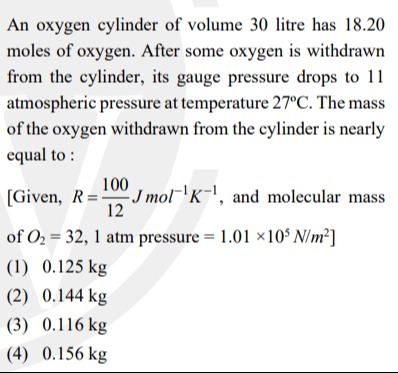
0.125 kg
0.144 kg
0.116 kg
0.156 kg
0.116 kg
Solution
The initial number of moles of oxygen in the cylinder is given as n1=18.20 moles. The volume of the cylinder is V=30 litre =30×10−3m3.
After some oxygen is withdrawn, the final gauge pressure is Pgauge,2=11 atm and the temperature is T2=27∘C=27+273=300K.
The absolute pressure is the sum of gauge pressure and atmospheric pressure. Assuming atmospheric pressure is 1 atm, the final absolute pressure is P2=Pgauge,2+Patm=11 atm+1 atm=12 atm.
We need to convert the pressure to SI units: P2=12×(1.01×105N/m2)=12.12×105N/m2.
The gas constant is given as R=12100Jmol−1K−1.
Using the ideal gas law for the final state, P2V=n2RT2, where n2 is the number of moles of oxygen remaining in the cylinder.
n2=RT2P2V
n2=(12100Jmol−1K−1)×(300K)(12.12×105N/m2)×(30×10−3m3)
n2=12100×30012.12×105×30×10−3
n2=123000012.12×300=3000012.12×300×12
n2=1012.12×12=12.12×1.2=14.544 moles.
The number of moles of oxygen withdrawn is Δn=n1−n2.
Δn=18.20 moles−14.544 moles=3.656 moles.
The molecular mass of oxygen (O2) is given as 32. This means the molar mass is 32 g/mol or 32×10−3 kg/mol.
The mass of oxygen withdrawn is Δm=Δn×M, where M is the molar mass.
Δm=3.656 moles×(32×10−3 kg/mol)
Δm=3.656×32×10−3 kg
Δm=116.992×10−3 kg
Δm=0.116992 kg.
This value is nearly equal to 0.117 kg, which is closest to 0.116 kg among the given options.
