Question
Question: An organic compound with the molecular formula \({C_8}{H_8}O\) forms 2,4 – DNP derivative, reduces T...
An organic compound with the molecular formula C8H8O forms 2,4 – DNP derivative, reduces Tollen’s reagent and undergoes Cannizzaro reaction. On vigorous oxidation, it gives 1,2-benzenedicarboxylic acid. The organic compound is:
(A) 2-ethylbenzaldehyde
(B) 2-methylbenzaldehyde
(C) acetophenone
(D) 3-methylbenzaldehyde
(E) phenylacetaldehyde
Solution
It is a carbonyl compound with no active hydrogen atoms or α-hydrogens. It has benzene parent carbon chain and oxidation product of 1,2-benzenedicarboxylic acid suggests that it has 2 substituents at 1 and 2 positions.
Complete step by step answer:
The molecular formula of the given compound is: C8H8O.
-Since the organic compound gives a 2,4 – DNP derivative, it means that it is a carbonyl compound because 2,4 – dinitrophenyl hydrazine (Brady’s reagent) reacts only with aldehydes and ketones to give a coloured precipitate.
Now we need to know whether the carbonyl group is of ketone or of aldehyde.
-This compound also reduces Tollen’s reagent and undergoes Cannizzaro reaction.
Tollen’s reagent is a solution of: silver nitrate (AgNO3) and ammonia (NH3) and is used for the detection of aldehydes. This reagent converts aldehydes to carboxylic acids. The general form of reaction by Tollen’s reagent is:
R−COH+2Ag++2OH−→R−COOH+2Ag+H2O
Silver mirror-like surfaces on the test tube indicate the presence of an aldehydic group. Due to this reason, Tollen’s test is also known as the silver mirror test.
Cannizzaro reaction: It is a reaction to check for the presence of aldehydes which do not have any active hydrogen or α-hydrogen. Here the aldehyde reacts with a strong base to undergo a redox (oxidation-reduction) reaction. This converts aldehydes into alcohol and carboxylic acid. The general form of Cannizzaro reaction is:
2R−CHONaOHH3O+R−CH2OH+R−COOH
So, reducing Tollen’s reagent and giving Cannizzaro reaction means that there are no α-hydrogen and the compound has an aldehydic group.
So, this tells us that the compound will be a benzene ring with 2 substituents: an aldehydic group (−CHO) and a methyl group (−CH3).
-Since vigorous oxidation of this compound gives 1,2-benzenedicarboxylic acid, so it proves that the aldehydic group (−CHO) and the methyl group (−CH3) are present at successive positions.
So, the compound will be: 2-methylbenzaldehyde and its structure will be:
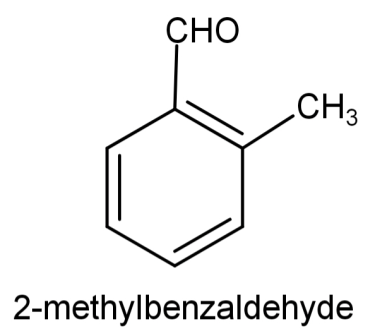
And the reactions involved will be:
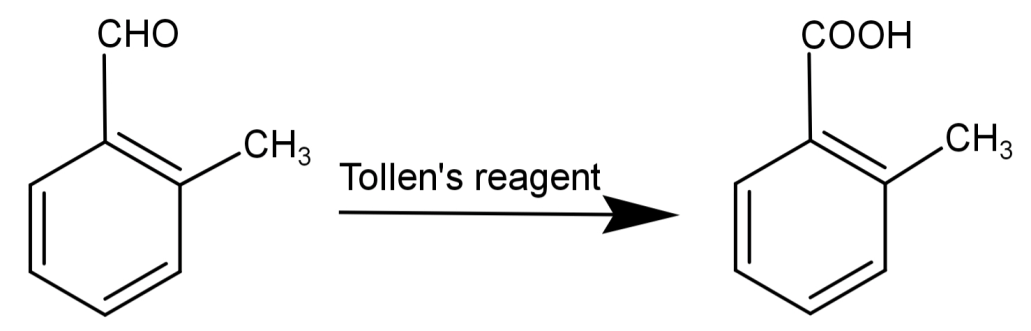
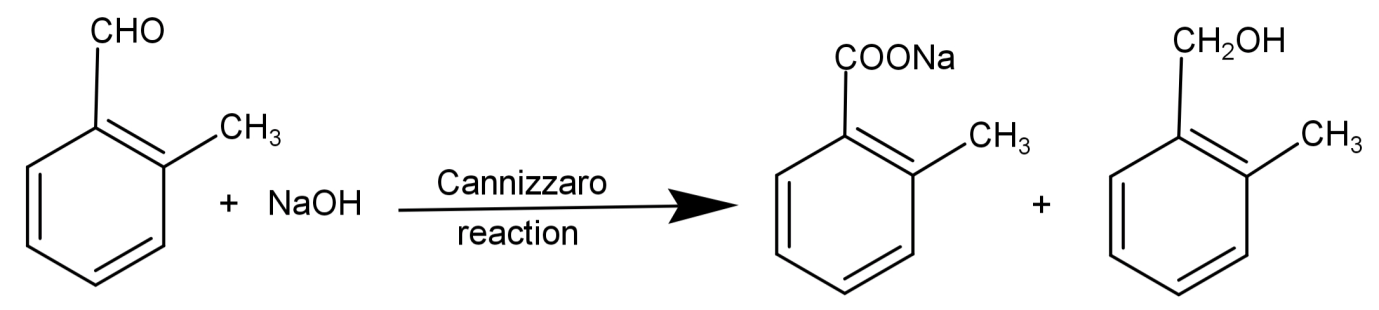
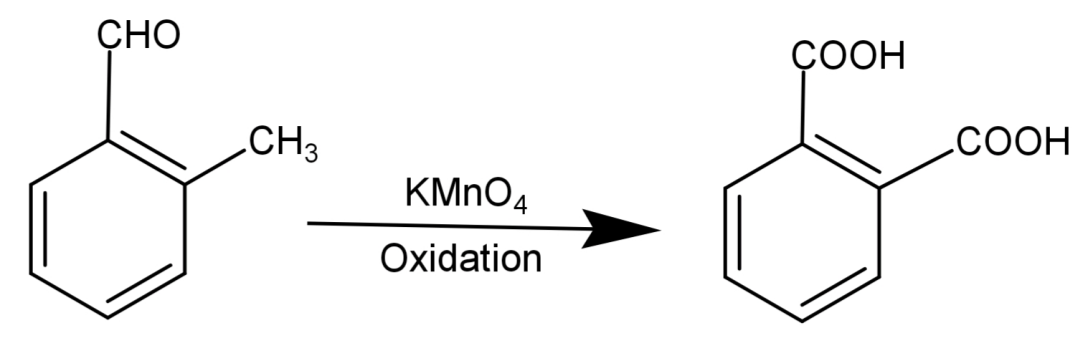
So, the correct answer is “Option B”.
Note: Active hydrogens or α-hydrogens are those H atoms which are attached to the carbon atom just adjacent to the functional group carbon or to the α-carbon atom. Also the alpha hydrogen of carbonyl groups are highly acidic due to stability of the anion which will be formed once the hydrogen atom is removed.
