Question
Question: An organic compound with molecular formula \({{\rm{C}}_{\rm{4}}}{{\rm{H}}_{{\rm{10}}}}{\rm{O}}\) doe...
An organic compound with molecular formula C4H10O does not react with sodium. With excess of HI it gives only one type of alkyl halide. The compound is:
A.C2H5OC2H5
B.
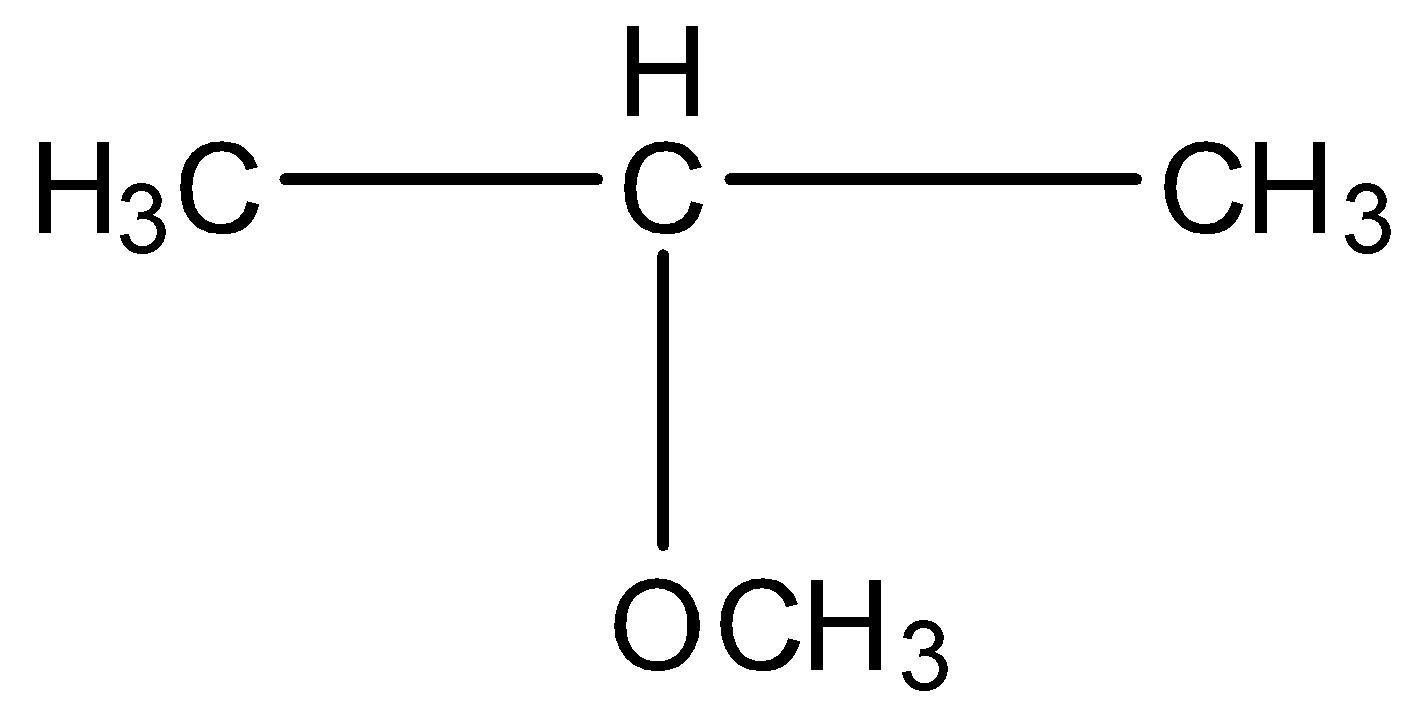
C.CH3CH2CH2OCH3
D.CH3CH2CH2CH2OH
Solution
know that an organic compound is the compound where there is a covalent link of carbon with other atoms like hydrogen, nitrogen, oxygen etc. Although in carbonates, carbides, cyanides carbon atoms are present but they are not classified as organic compounds.
Complete step by step answer:
Here, the molecular formula of the compound is C4H10O. The O atom in the compound indicates that there may be a presence of alcohol, carbonyl group or ether. But compounds in the options are ether and alcohol. Also, given that the compound does not react with sodium. We know that alcohol reacts with sodium to form sodium alkoxide (salt) that means the compound must be ether.
Now, we have to identify the correct ether whose molecular formula is C4H10O. Another information given is that the compound when undergoing reaction with excess of HI gives one type of alkyl halide. For this, both the alkyl groups bonded to the oxygen atom must be the same. So, the compound is C2H5OC2H5. The chemical reaction can be shown as below:
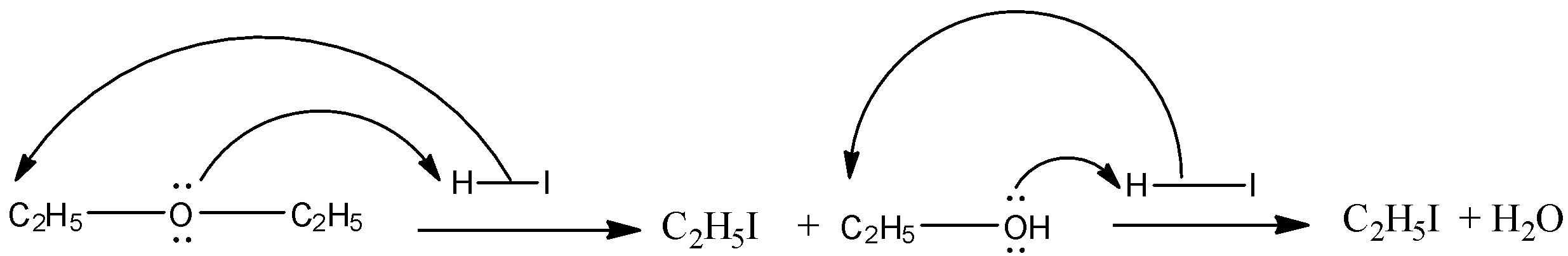
So, when C2H5OC2H5 reacts with excess HI, only one type of alkyl halide(C2H5I)produces.
So, the correct answer is Option A.
Additional Information:
Functional groups are atoms or groups of atoms that decide the chemical properties of an organic compound. Some functional groups are alcohol, aldehyde, carboxylic acid, ether, amide etc. The structure of ether is R-O-R (R is alkyl group). If the two alkyl groups in ether are same, the ether is termed as symmetrical ether and if alkyl groups are different then the ether is termed as unsymmetrical ether.
Note:
There are many methods for preparation of ether, such as, Williamson synthesis, dehydration of alcohol etc. Williamson synthesis is the method used in the laboratory for preparation of symmetrical and unsymmetrical ether. In this method, an alkyl halide undergoes reaction with sodium alkoxide.
R−X+R−O−Na→R−O−R+NaX
Here, R is an alkyl group.
