Question
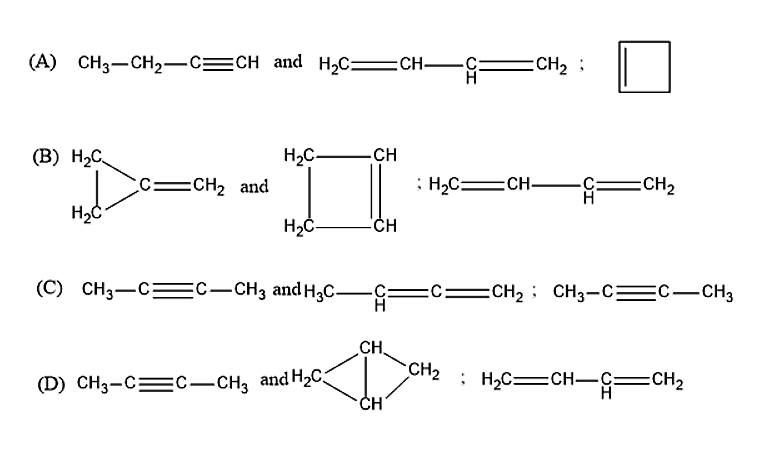
Question: An organic compound of molecular formula \[{C_4}{H_6}\] (A), forms precipitate with ammoniacal silve...
An organic compound of molecular formula C4H6 (A), forms precipitate with ammoniacal silver nitrate and ammoniacal cuprous chloride. ‘A’ has an isomer ‘B’, one mole of which reacts with one mole of Br2 to form 1,4-dibromo-2-butene. Another isomer of ‘A’ is ‘C’, 1 mole of C reacts with only 1 mole of Br2to give vicinal dibromide. A, B & C.
Solution
First thing we have to do in this reaction is to find the compound A from the molecular formula given. Then only we will be able to carry out the further reaction and then find the compound B and C.
Complete answer:
An organic compound is given which has a molecular formula of C4H6. When the compound A reacts with the ammoniacal silver nitrate and ammoniacal cuprous chloride, it will form a precipitate. Hence, the compound A was found to be 1-Butyne.
1-Butyne is having an isomer, i.e., the compound B. One mole of the compound B will react with one mole of the bromine molecule to form 1,4-Dibromo-2-butene. Therefore, the compound B was found to be 1,4-Butadiene.
Butyne is also having another isomer other than 1,4-Butadiene, i.e., the compound C. One mole of the compound C will react with one mole of the Bromine molecule to give vicinal dibromide. Therefore, the compound C is found to be 1-Cyclobutene.
Therefore, the correct answer is option (A).
Note:
We should always be careful while finding the compound A, because by knowing it only we would be able to carry out the rest of the reaction. If we make a mistake in finding it, then there is a chance that the whole reaction can go wrong.
