Question
Question: An organic compound having molecular mass 60 is found to contain C = 20%, H = 6.67% and N = 46.67%, ...
An organic compound having molecular mass 60 is found to contain C = 20%, H = 6.67% and N = 46.67%, while rest is oxygen. On heating, it gives NH3 along with a solid residue. The solid residue gives violet colour with alkaline copper sulphate solution. The compound is:
A.CH3NCO
B.CH3CONH2
C.(NH2)2CO
D.CH3CH2CONH2
Solution
In this question, we are given the percentage of each element present in the compound. With the help of the percentage, and atomic mass of the element find out the number of atoms present in the compound. It will help to derive the molecular formula of the compound.
Complete step by step answer:
-Now, we know the percentage of carbon, hydrogen, and nitrogen present in the compound.
-First, we will calculate the percentage of oxygen present in the compound. Thus, it can be written as:
Total percentage = C% + H% + N% + O%
100% = 20% + 6.67% + 46.67% + O%
So, the percentage of oxygen is 26.66 %
-Now, we will calculate the ratio of the number of atoms present in the compound, i.e.
C:H:N:O=1220:16.67:1446.67:1626.66
Here, 12, 1, 14, and 16 represent the atomic mass of carbon, hydrogen, nitrogen, and oxygen respectively.
-So, after calculating we got;
C:H:N:O=1:4:2:1
-Therefore, we can say that compounds contain the number of atoms as mentioned above. The formula can be written as: CH4N2O
-It is the empirical formula of the compound.
-We have got the empirical formula for the compound. The empirical weight of the compound is 60 g.
-According to the question, the molecular weight of the compound is 60 g.
Therefore, Molecular weight = n (Empirical weight)
-Here, we get n = 1
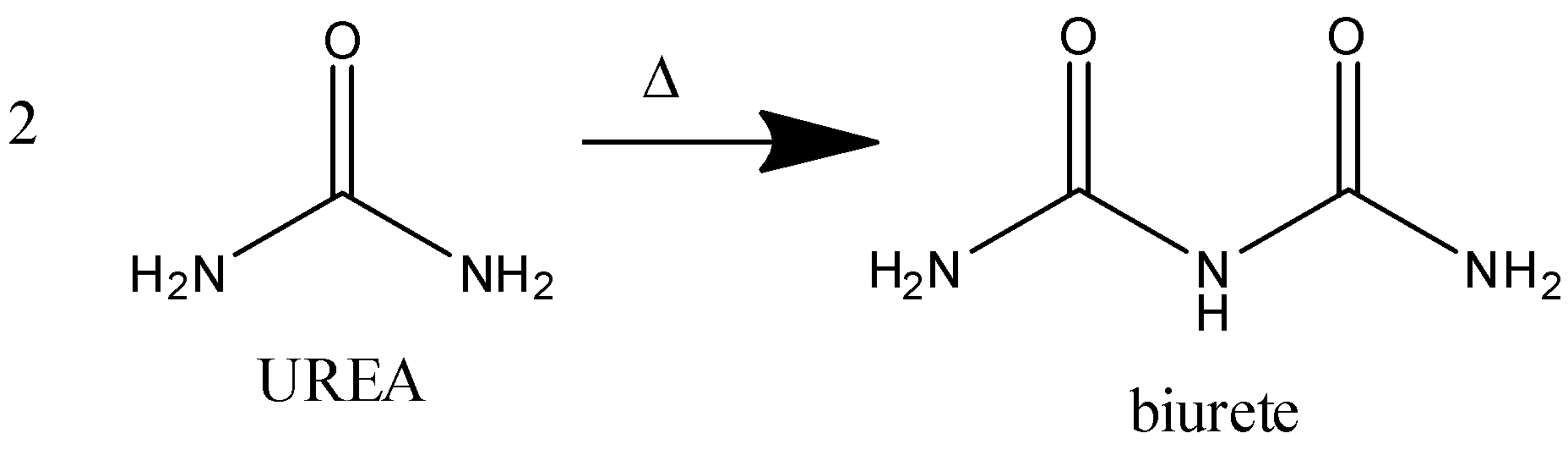
-We know that the biuret test NH3 is released. It gives a violet color
-Thus, we can say that the molecular formula of the compound is (NH2)2CO.
-Hence, the correct option is C.
Note:
Molecular formula gives the chemical proportion of atoms that constitutes a particular chemical compound. The molecular formula can also be found out by dividing the empirical molecular mass by 2. From the molecular formula of water, it can be concluded that a molecule consists of 2 Hydrogen atoms and 1 oxygen atom. The empirical formula gives the whole number ratio of atoms in a compound.
