Question
Question: An organic compound having molecular formula \({{C}_{3}}{{H}_{6}}O\) gives orange-red precipitate wi...
An organic compound having molecular formula C3H6O gives orange-red precipitate with 2,4-dinitrophenyl hydrazine, but does not reduce Tollens reagent. Give IUPAC the name and structural formula of the compound.
Solution
Both aldehydes and ketones give orange-red precipitates on reaction with 2,4-dinitrophenyl hydrazine, which is commonly known as DNP, to form 2,4-dinitrophenylhydrazones.
Tollen’s reagent is an ammoniacal solution of silver nitrate. Tollen’s reagent is reduced to metallic silver by aldehydes. Ketones do not react with Tollen’s reagent.
Complete answer:
Let us see what we have been given about the compound.
Molecular formula of the compound is C3H6O.
It forms orange-red precipitates with 2,4-dinitrophenyl hydrazine.
It does not reduce Tollens reagent.
Let us try to deduce the structure of the organic compound with this information.
We know that both aldehydes and ketones react with 2,4-dinitrophenyl hydrazine (DNP) to form orange-red precipitates of 2,4-dinitrophenylhydrazones.
Structure of aldehyde and ketone with the molecular formula C3H6O.
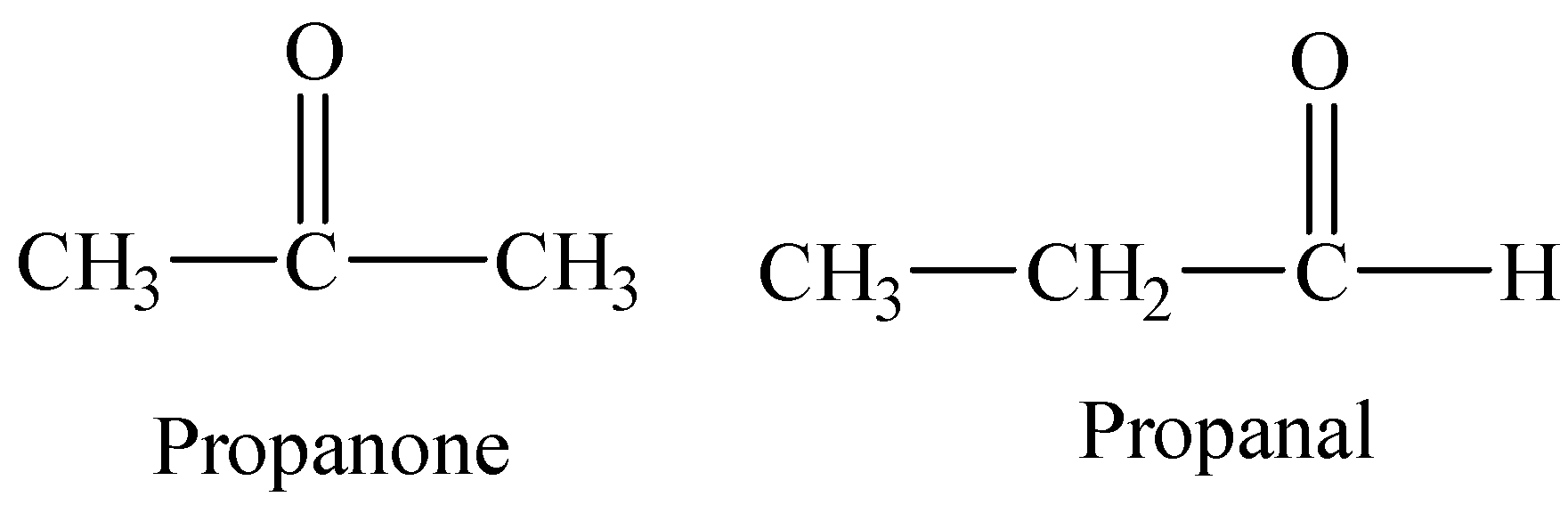
Reactions of propanal and propanone with DNP are given below:
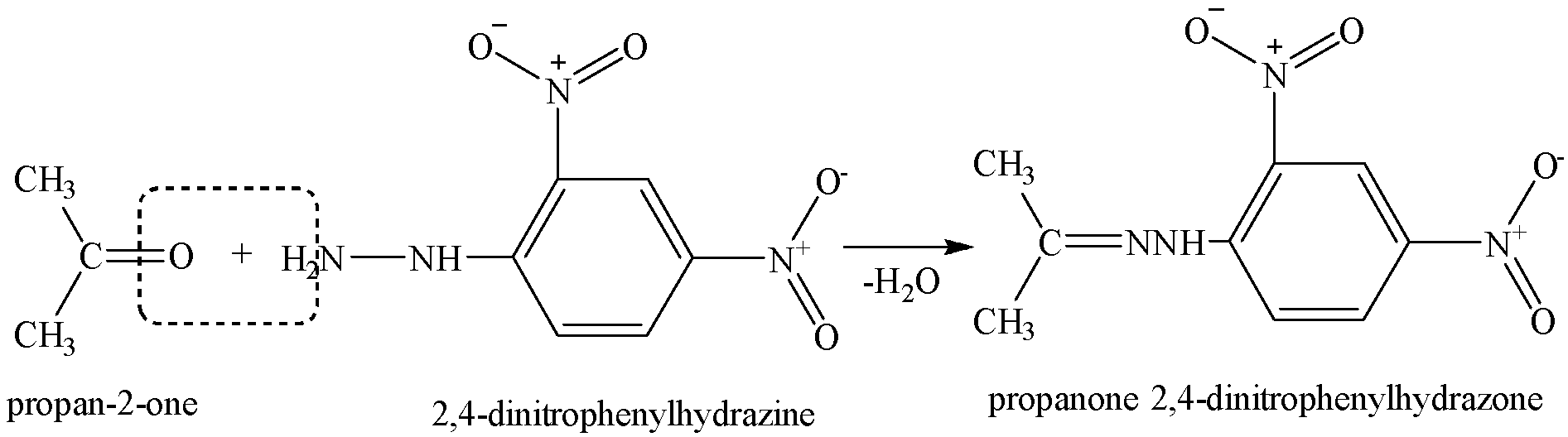
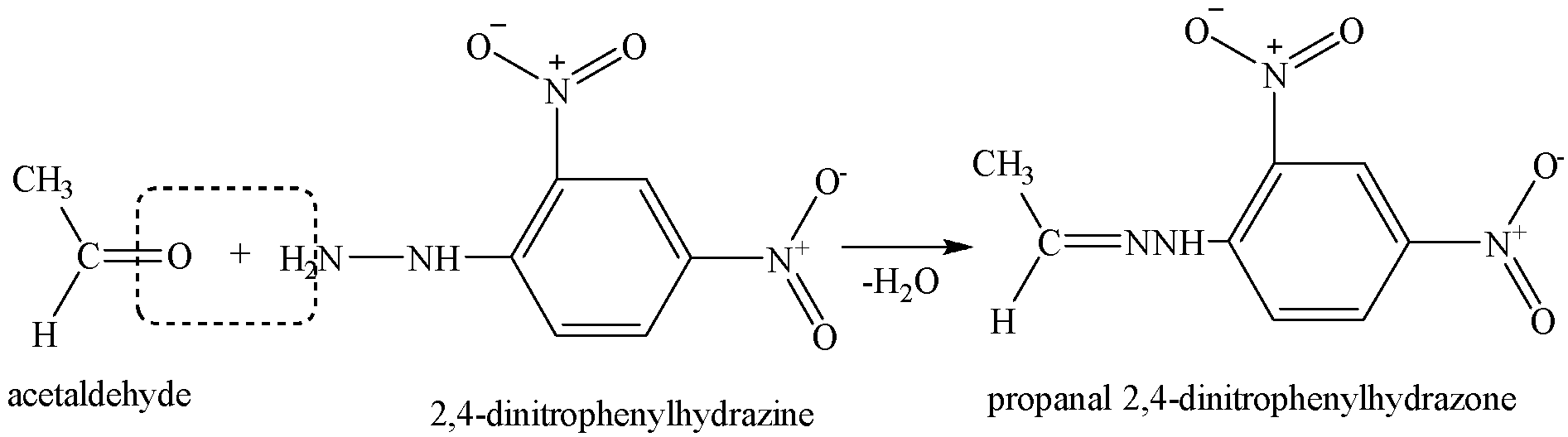
Thus, the given compound could be aldehyde or ketone as both an aldehyde and ketone can be represented by the same molecular formula C3H6O.
But it is given that the compound does not reduce Tollens reagent. We know that only aldehydes react with Tollen’s reagent and reduce it to silver metal.
Therefore, out of propanal and propanone, only propanal reduces Tollen’s reagent when heated. The reaction of propanal with Tollen’s reagent is given below:CH3CH2CHO+2[Ag(NH3)2]++3OH−ΔCH3CH2COO−+2Ag↓+4NH3+2H2O
CH3COCH3+2[Ag(NH3)2]++3OH−Δ No reaction
Therefore, the compound with molecular formula C3H6O showing reactions with 2, 4-dinitrophenyl hydrazine and Tollen’s reagent is an aldehyde.
Structural formula of the compound and its IUPAC name is:
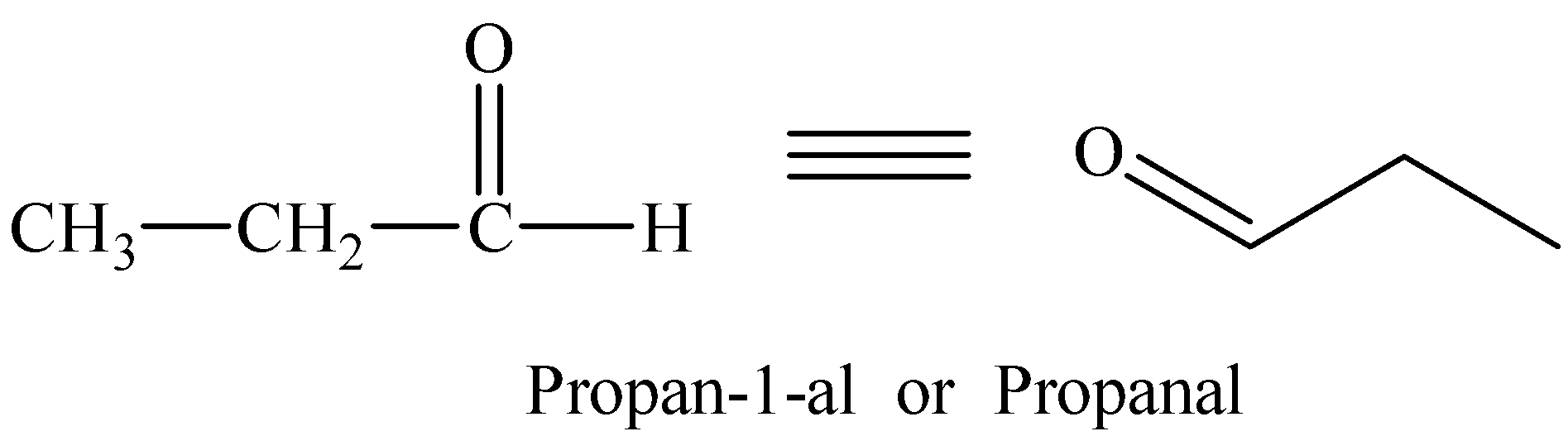
Note:
Note that while writing the IUPAC name of a ketone, a suffix -one is added. Always remember that Tollen’s reagent is only reduced by both aliphatic and aromatic aldehydes. Also note that no other isomeric structure of aldehyde can be written for the given molecular formula.
