Question
Question: An organic compound contains \(69.77%\) carbon, \(11.63%\) hydrogen and rest oxygen. The molecular m...
An organic compound contains 69.77 carbon, 11.63 hydrogen and rest oxygen. The molecular mass of the compound is 86. It does not reduce Tollens reagent but forms an additional compound with sodium hydrogen sulphite and gives positive iodoform test. On vigorous oxidation, it gives ethanoic and propanoic acid. Write the possible structure of the compound.
Solution
Hint The percentage composition will provide the ratio in which the atoms in the compound are present, giving the empirical formula. This is further related to the molecular formula by the n-factor.
Complete step by step answer:
The percentage composition of the components is given as follows:
Percentage of carbon =69.77
Percentage of hydrogen =11.63
Percentage of oxygen =(100−69.77−11.63)=18.6
So, the molar ratio of carbon, hydrogen and oxygen in the compound is given by:
C:H:O=1269.77:111.63:1618.6
Then, on simplifying, we get, C:H:O=5:10:1
So, the empirical formula for the compound will now be C5H10Oand the empirical formula mass will be (5×12+10×1+1×16)=86g.
The molecular mass also given equal to 86g. So, the n-factor =empirical formula massmolecularmass=8686=1 .
Therefore, the molecular formula of the compound is C5H10O.
Now, the structure of the compound can be detrained, following through the tests it satisfies, which are as follows:
- Firstly, the Tollens test, will distinguish between the aldehydes and ketones. As the Tollens reagent is not reduced by the compound, so it will not be an aldehyde. We thus get a ketone.
- Further, the compound reacts with sodium hydrogen sulphite giving a positive iodoform test. Thus, the ketone compound is rather a methyl ketone compound.
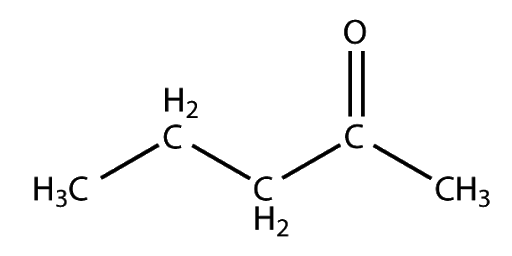
- This methyl ketone on vigorous oxidation gives ethanoic and propanoic acid.so, the compound will be Pentan-2-one.
Reaction as follows: Pentan-2-oneCH3COCH2CH2CH3→Ethanoic acidCH3COOH+Propanoic acidCH3CH2COOH
Therefore, the possible structure of Pentan-2-one is shown below:
Note: The n-factor gives the ratio of the atoms in the molecular formula as an integer multiple of the atoms in the empirical formula, which helps to obtain the actual molecular formula of the compound.
