Question
Question: An organic compound contains \(66\% \) carbon, \(13.3\% \) hydrogen and the remaining is oxygen. Its...
An organic compound contains 66% carbon, 13.3% hydrogen and the remaining is oxygen. Its vapour density is 37 . The possible number of isomers of all types of the compound is:
A.6
B.7
C.5
D.8
Solution
In this question, in order to find the number of possible isotopes, first we need to find the molecular formula of the organic compound. Molecular formulas can be easily found using the percentage mass of elements in compounds and its molecular mass.
Complete step by step answer:
Given, vapour density of the compound is 37. Molecular mass can be find out from vapour density using the formula,
Molecular mass = Vapour density ×2
Hence molecular mass of given compound is,
Molecular mass =37×2=74g/mol
Given the organic compound contains 66% carbon, 13.3% hydrogen and the remaining is oxygen. Let x be the weight of carbon in the compound. Then we can write,
74x×100=66 x=10066×74=48.84g
The compound contains 48.84g of carbon. Atomic mass of one carbon atom is 12g. Hence number of carbons in the compound will be,
1248.84=4.07
We can round it to 4 .
Similarly we can find out the number of hydrogens and oxygens present in the molecule.
Let y be the weight of hydrogens in the compound. Then we can write,
74y×100=13.3 y=10013.3×74=9.842g
The compound contains 9.842g of hydrogen. Atomic mass of a hydrogen atom is 1g. Hence number of hydrogens in the compound will be,
19.842=9.842
We can round it to 10 .
Let z be the weight of oxygen in the compound. The percentage of oxygen in the compound will be,
20.7% . Then we can write,
74z×100=20.7 z=10020.7×74=15.318g
The compound contains 15.318g of oxygen. Atomic mass of an oxygen atom is 16g. Hence number of oxygens in the compound will be,
1615.318=0.9574
We can round it to 1 .
Hence the molecular formula of the compound is C4H10O . Let us draw all the possible structures with this molecular formula. The possible structures are,
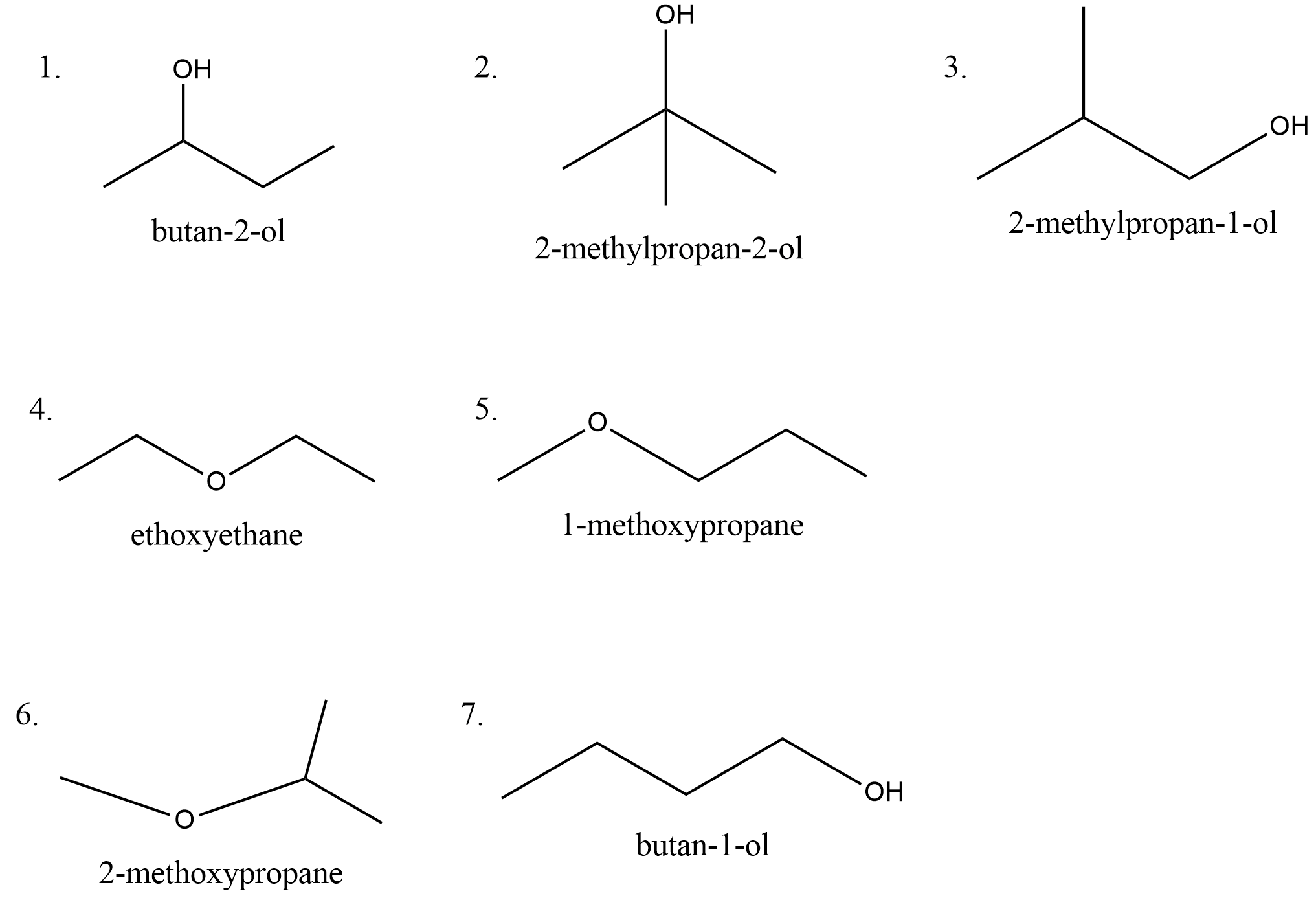
There are seven isomers possible which contain alcohols and ethers.
Hence the correct option is B.
Note:
Since the compound contains one oxygen atom, we may think that we can draw aldehyde and ketone as one of the isomers. But if we try to draw and aldehyde or ketone with 4 carbons, only 8 hydrogens are required. We have 10 hydrogens in the molecular formula. Hence we don’t have an aldehyde or ketone as one of the isomers.
