Question
Question: An organic compound \({C_4}{H_{10}}O{\text{ (X)}}\) on reaction with \({I_2}/red - P\) gives \({C_4}...
An organic compound C4H10O (X) on reaction with I2/red−P gives C4H9I which on further reaction with AgNO2 gives C4H9NO2 (Y). Y on treatment with HNO3 form a blue solution which in turns to red on making the solution slightly alkaline. The possible identity of X is:
A. 1-butanol
B. 2 methyl-1-pentanol
C. 2-butanol
D. Either 1 or 2
Solution
Follow each step of the question with options to reach the solution. Red phosphorus is a hard crystalline solid without any smell and is a bad conductor of electricity. Chemically being less reactive than white phosphorus, red phosphorus reacts with halogens, sulphur and alkali metals only when heated forms their corresponding salts and also red phosphorus does not react with caustic alkalis.
Complete answer:
The organic compound (X) given is C4H10O.
1-butanol on reaction with I2/red−P gives Butyl iodide. (given C4H9I)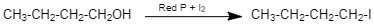
Silver nitrite is a covalent compound and the bond between Ag-O is covalent.Therefore it does not have a negative charge on the oxygen atom. Hence, the nucleophilic attack occurs through the lone pair on nitrogen forming nitroalkanes. So, When this haloalkane (1-iodobutane) is treated with silver nitrite, (AgNO2), the halogen atom is replaced by nitro group (−NO2) to give Nitroalkane (1-Nitrobutane). (Given C4H9NO2 is (Y))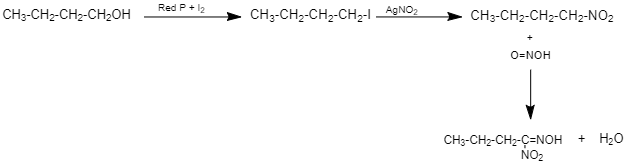
This 1-Nitrobutane on treatment with nitrous acid forms Nitrolic acid, (a blue coloured solution) which dissolves in alkali to give a red coloured solution.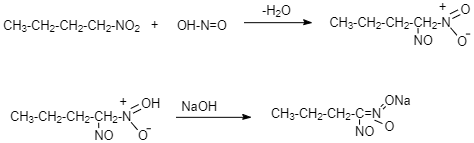
The answer cannot be 2-butanol as 2-Nitrobutane reacts with nitrous acid to produce Pseudo nitrole which gives blue colour with alkali.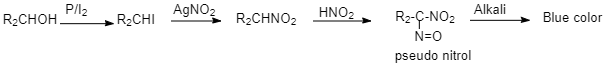
So the organic compound(X) is 1-butanol
Therefore the correct answer is option (A).
Note:
The Victor Meyer apparatus is the usual laboratory method of determining the molecular weight of a volatile liquid.It was developed by Viktor Meyer, who spelled his name Victor in publications at the time of its development. In this method, a known mass of a volatile solid or liquid under examination is converted into its vapour form by heating in a Victor Meyer's tube.
