Question
Question: An organic compound ( \[{{C}_{3}}{{H}_{9}}N\]) (A) when treated with nitrous acid, gave an alcohol a...
An organic compound ( C3H9N) (A) when treated with nitrous acid, gave an alcohol and N2 gas was evolved. (A) on warming with CHCl3 and caustic potash gave (C) which on reduction gave isopropylmethylamine. Predict the structure of (A).
(A)
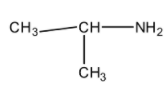
(B)
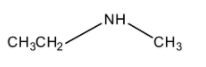
(C)
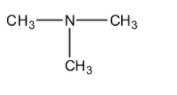
(D)
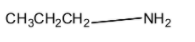
Solution
It is to be remembered that when an amine reacts with HNO2 and gives an alcohol along with nitrogen gas, then the amine which reacted must be a primary amine.
Complete answer:
First, let us take the compound C3H9N and we have to determine whether it is primary, secondary or tertiary amine. Now when the compound reacts with HNO2 then we observe that nitrogen gas evolves and an alcohol is formed. This reaction is only given by primary amines so the structure of the compound can be predicted.
We are given that the compound has 3 atoms of C, 1 atom of N and 9 atoms of H. Also, it is a primary amine. So, C3H7NH2 can be a possible structure according to the test.
For solving the next part of the question , it is given that this amine on warming with CHCl3 and caustic potash and reduction gave isopropyl methyl amine, that means that the compound asked for can successfully undergo iodoform test.
So the final structure of the organic compound comes to be:
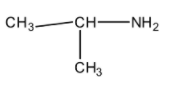 which is called isopropylmethylamine.
which is called isopropylmethylamine.
If you count the number of atoms, it has exactly 3 C, 9 H and 1 N which matches with the criteria of the question.
Hence, Option A is our answer.
Note:
You need to remember that iodoform reaction is used to determine the presence of methyl ketone. In this case, it was present and hence, it reacted. Always look up to the solutions in the question for hints; in this case you could have eliminated options A and B as they are not primary amines.
