Question
Question: An organic compound (A) with molecular formula \({C_7}{H_8}O\) dissolves in \(NaOH\) and gives chara...
An organic compound (A) with molecular formula C7H8O dissolves in NaOH and gives characteristic color with FeCl3 . On treatment with Br2 , it gives a tribromo product C7H5OBr3 . The compound is –
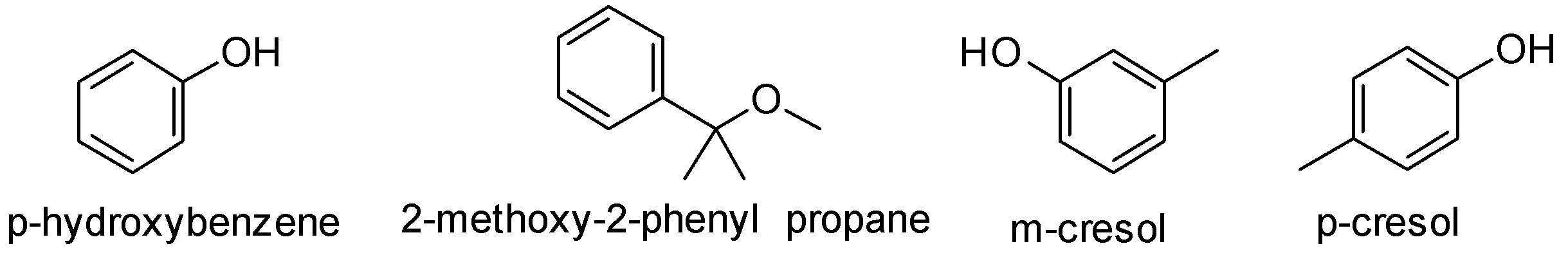
A. p-hydroxybenzene
B. 2-methoxy-2-phenyl propane
C. m-cresol
D. p-cresol
Solution
T First try to find the type of compound from the given formula. FeCl3 is used to detect the presence of phenol in the compound. The number of sites of unsaturation can also be found out. Reaction of bromine to give a trisubstituted product is an example of electrophilic substitution reaction.
Complete step by step answer:
The structures of the given compounds are-
The formula given suggests that there are four sites of unsaturation in the given compound A. It can be calculated as-
Formula- C7H8O
According to the 2n+2 rule,
n=7 , the saturated compound will have formula C7H16 .Here we do not count anything for oxygen atoms. So there are eight hydrogen atoms less. It means that there are four sites of unsaturation in the given sample compound. We could suggest the presence of an aromatic ring that has four sites of unsaturation.
Compound (A) gives color with FeCl3 . It is used to detect the presence of phenols in the sample. So this confirms that our given compound (A) is a phenol. 2-methoxy-2-phenylpropane is an ether. Also it has eleven carbon atoms while compound (A) has only six carbon atoms. So option B cannot be the right answer and can be ruled out.
Also p-hydroxybenzene has only six carbon atoms while the given compound (A) has seven carbon atoms. So option A is not the correct answer.
Compound (A) on reaction with bromine gives a tribromo product. Phenols undergo electrophilic substitution reactions. The −OH group activates the benzene ring and makes the ortho, para position electron rich due to resonance effect. Hence the incoming group is directed to ortho and para position. The tribromo product suggests that the three bromine atoms must be present at the ortho and para position. m-cresol has methyl group at meta position and the ortho and para positions are vacant. So it can form a tribromo product.
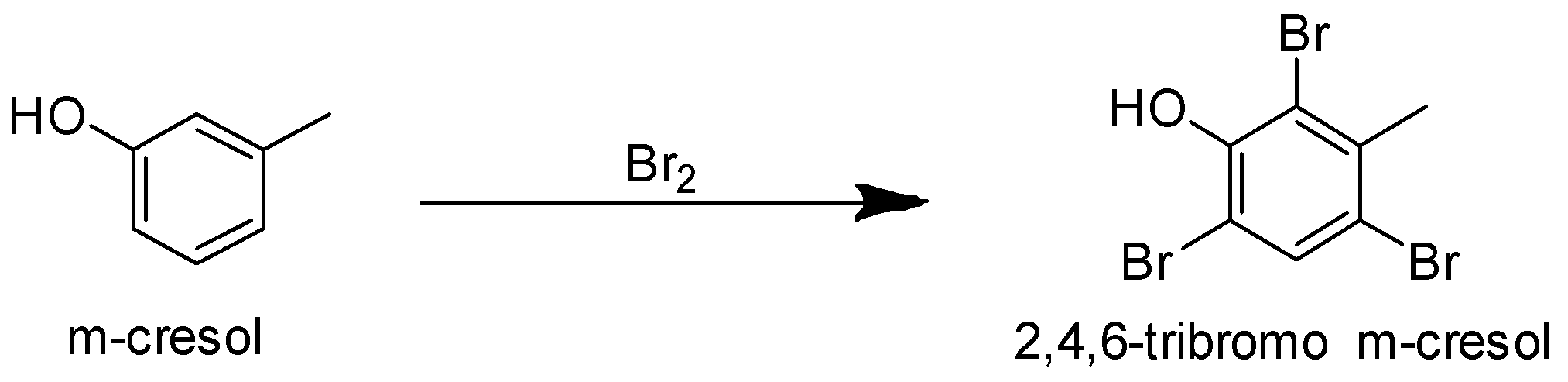
p-cresol cannot form a tribromo product with bromine as it has methyl group at its para position. So the para position is blocked for the incoming bromine atom. Bromine can be substituted only at the ortho position.
Hence correct answer is optionC.
Note:
Phenols undergo electrophilic substitution reactions at the ortho and para positions since the −OH group is an activating group.
FeCl3 is used as a test for detection of phenols. If phenols are present in the sample they give a characteristic color.
Halogens and −OH groups are electron releasing groups. They give ortho-para substituted products.
