Question
Question: An organic compound A with a molecular formula \[{{C}_{2}}{{H}_{7}}N\] on reaction with nitrous acid...
An organic compound A with a molecular formula C2H7N on reaction with nitrous acid gives a compound B. B on controlled gives compound C. C reduces Tollen’s reagent o give silver mirror and D. B reacts with D in the presence of concentrated sulphuric acid to give sweet smelling compound E. Identify A, B, C, D and E. Give the reaction of C with ammonia?
Solution
Hint: Conversion of an ammonia like odour organic compound to the sweet smelling organic compound. In between silver is also produced. A has an ammonia like smell and B has an alcoholic smell. On controlled oxidation of B we get a carbonyl compound C and this compound can reduce tollen’s reagent and gets converted to an acid D. On esterification of D and B we get a sweet smelling compound E.
Complete step-by-step answer:
It is given that the molecular formula of A is C2H7N .
Let us consider A as ethyl amine C2H5NH2.
A reaction with nitrous acid gives ethanol or B.
AC2H5NH2HNO3BC2H5OH
Next step is B (ethanol) on controlled oxidation gives C (acetaldehyde).
BC2H5OHcontrolledoxidationCCH3CHO
C, that is acetaldehyde reduces Tollen’s reagent. The produces are silver mirror and D (acetic acid)
CCH3CHO+2[Ag(NH3)2]++3OH−heatDCH3COO−+2Ag↓+2H2O+4NH3
D or acetic acid and ethanol reacts in the presence of sulphuric acid to give the sweet smelling compound of an ester ethyl acetate.
DCH3COOH+C2H5OHconcH2SO4ECH3COOC2H5
This is the conversion of a primary amine to an ester.
Thus, A-C2H5NH2, B-C2H5OH, C-CH3CHO, D-CH3COOH, E-CH3COOC2H5
C reacts with ammonia to give acetaldehyde ammonia.
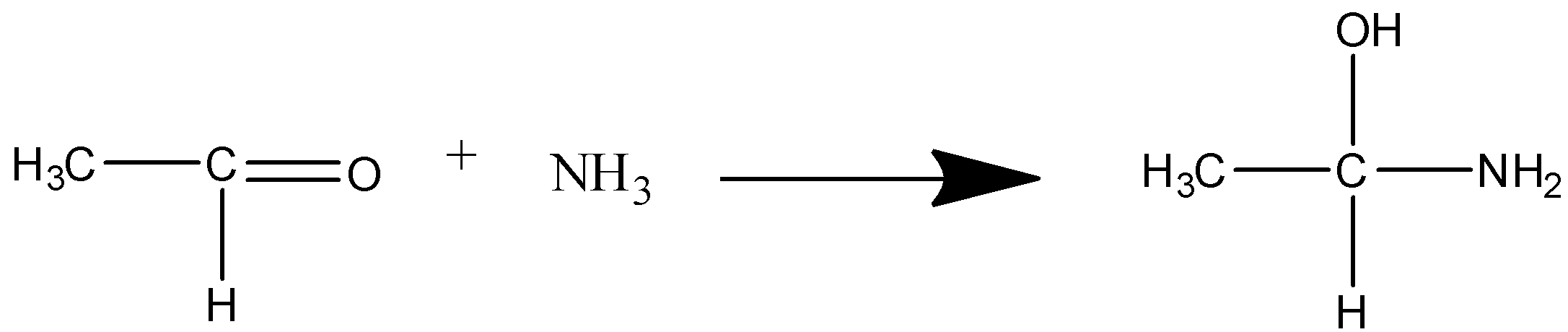
Ammonia attacks the double bond O of the aldehyde and converts the aldehyde to acetaldehyde ammonia.
Additional Information: Conversions are the easy steps or hacks to convert one organic compound to another. It can be single step or multi step. Conversion schemes cannot be used as such in organic synthesis, when actually synthesising various parameters is involved. We should have a general idea of all the organic reactions while developing a conversion scheme.
Note: Whenever nitrogen is present in the given molecular formula without oxygen, then the only possibilities are derivatives of amines or nitrile compounds. Only aldehydes reduce Tollen’s reagent to silver, ketones do not.
