Question
Question: An organic compound (A) of molecular formula \[{{C}_{3}}{{H}_{8}}O\] gives turbidity within 5-10 min...
An organic compound (A) of molecular formula C3H8O gives turbidity within 5-10 min on reaction with anhydrous ZnCl2/HCl. Compound (A) on treatment with sodium hypochlorite gives a carbonyl compound (B) which on further chlorination gives compound (C) of molecular formula C3H3OCl3. Identify (A), (B), and (C). Explain the reactions.
Solution
Isopropyl alcohol is a colourless and flammable liquid. When this compound reacts with sodium hypochlorite, it gives ketone group which on further chlorination shows ester group formation.
Complete answer:
- In the question, we have the molecular formula of the compound (A), i.e. C3H8O. So, the molecular structure would be
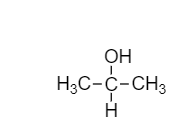
- This is our compound (A) Methoxy ethane or Isopropyl alcohol.
-As we know, this compound gives turbidity within 5-10 min on reaction with anhydrous ZnCl2/HCl. On treatment with Sodium hypochlorite NaOCl, we get compound (B)
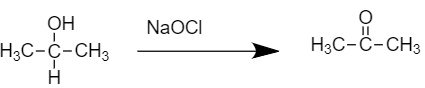
- The resulting product is our compound (B) Acetone.
- Compound (B), on further chlorination gives compound (C) with the molecular formulaC3H3OCl3. So, the reaction would be as shown below
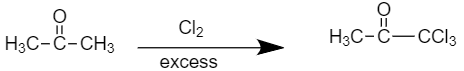
The final product is our compound (C) Methylchloroglyoxylate.
Additional information:
Sodium hypochlorite, on ingestion, can show minor damage to the esophagus, causing first-degree burns with hyperemia and edema of the mucosa. ZnCl2 is a water soluble, highly hygroscopic compound. It finds wide variety under the textile industry and chemical synthesis.
Note:
Sodium hypochlorite is commonly known as bleach. It is frequently used as a disinfecting agent and very effective for the disinfection of viruses, bacteria, fungi, and mycobacterium. However, sodium hypochlorite is NOT effective in the disinfection of bacterial spores and prions.
