Question
Question: An organic compound \[A\left( {{C_4}{H_9}Cl} \right)\] on reaction with \[Na\]\[/\]diethyl ether giv...
An organic compound A(C4H9Cl) on reaction with Na$$$$/diethyl ether gives a hydrocarbon which on monochlorination gives only one chloro derivative then, A is:
A.isobutylchloride
B.secondarybutylchloride
C.tertiarybutylchloride
D.n−butylchloride
Solution
We should know the concept of Wurtz’s reaction. It involves the reaction of alkyl halides with Na in ethanol to form higher alkanes. This compound is sparingly soluble in water, miscible with alcohol and water. tetramethylbutane
Complete step by step answer:
The organic chemical reaction where Sodium metal reacts with two alkyl halides in the presence of dry ether to form alkane is called Wurtz’s reaction. The term hydrocarbon indicates symmetrical hydrocarbon Alkane.
The general equation of Wurtz’s reaction is shown below,
R−X+2Na+R−XdryetherR−R+2NaX
Let us see another example of Wurtz’s reaction,
The reaction between methyl bromide and sodium in presence of anhydrous ether gives Ethane and byproduct.
2CH3Br+2Na→C2H6+2NaBr
The given organic compound A(C4H9Cl) on reaction with Sodium or diethyl ether gives a hydrocarbon which is 2,2,3,3− tetramethylbutane. Its structure is given below,
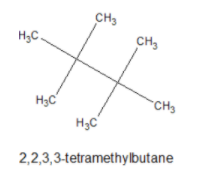
Chlorine replaces the one type of H atom in this compound and gives only a monochloro derivative. So the compound A(C4H9Cl) is tertiary butyl chloride.
Therefore the correct answer is option C.
ADDITIONAL INFORMATION:
The mechanism of Wurtz’s reaction involves a free radical species which is a part of metal − halogen exchange.(CH3)3CCl is the chemical formula of tertiary butyl chloride.
Dry ether is a very good poly aprotic solvent. Dry ether is completely free from water.
Note:
Methane cannot be prepared via Wurtz’s reaction since the product of this reaction must have at least two carbon atoms. Tertiary alkyl halides do not respond to this reaction. Only symmetric alkane can be synthesized through this method. We should note that sodium metal used in Wurtz’s reaction is highly reactive, so we need a solvent which does not react with sodium metal.
