Question
Question: An organic Compound (A) is widely used as a preservative in a pickle and has a molecular formula \[{...
An organic Compound (A) is widely used as a preservative in a pickle and has a molecular formula C2H4O2. This compound reacts with ethanol to form a sweet smelling compound (B)
A.Identify the compounds A and B
B.Name the process and write the corresponding chemical equation
Solution
It is a carboxylic acid to which methyl group is attached.The other name of compound (A) formed is Vinegar, and vinegar is used as preservative in pickle. Molecular Weight of acetic acid is 60.052g/mol.
Complete step by step answer:
We know Ethanoic acid is used as a solvent and as a Vinegar in the food Industry, vinegar is the dilute solution of acetic acid. Its structural formula is CH3COOH, therefore compound (A) is Acetic acid.
Also when compound (A) reacts with Ethanol it produces sweet smelling compound which is an ester i.e.,
{\text{CH3COOH}}$$$$ + {{\text{C}}_{\text{2}}}{{\text{H}}_{\text{5}}}{\text{OH}}\mathop \leftrightarrows \limits^{{H^ + }} {\text{C}}{{\text{H}}_{\text{3}}}{\text{COO}}{{\text{C}}_{\text{2}}}{{\text{H}}_{\text{5}}} + {{\text{H}}_2}{\text{O}}
Hence the compound (B) formed is Ethyl Ethanoate.
To get ethanoic acid from ethyl ethanoate hydrolysis process is followed with the chemical equation
CH3COOC2H5 + H2OH + CH3COOH + C2H5OH
Additional Information- Ethanoic acid, also called acetic acid as its common name. . It is used in making a polymer called acetate rayon.The structure of ethanoic acid is
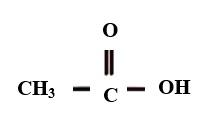
Molecular Weight: 62.037 molg|
---|---
Ethanoic acid is when it gets undiluted it is known as glacial acid.
Note:
Ethanoic acid, also called acetic acid as its common name, is a weak acid distinguishable by its sharp, vinegar like smell .
In Chemistry, after every reaction has thus formed will always give a foul smell, Only Ester is a compound in general which gives a fruity smell/pleasant smell. It is also used in the food industry as an acidity regulator.
